There is a timelessness to minerals and fossils that appeals to many of us. These marvellous treasures emerge against the most outrageous odds, first in their formation and then in their endurance through eons of sedimentation, metamorphosis, uplift and weathering.
I could happily spend hours marvelling at good mineral collections and the colours and complexity of the crystal specimens, but fossils have always held the most fascination for me. And I can easily name my favourite to date – an opalised fossil that I first saw in the wonderful Royal Tyrrell dinosaur museum in Drumheller, a town located at the bottom of a ditch in the Alberta Badlands of Canada.
Closer to home, the South Australian Museum recently showcased an outstanding collection of opals and opalised fossils, which is particularly appropriate given that Australia produces 95% of the world’s precious opals. Opals are found worldwide but precious opal is largely Australian.
Perhaps surprisingly, opals aren’t minerals by formal definition. Minerals are crystalline, while precious opal is composed of an orderly lattice of amorphous silica spheres, approximately 130–300 nanometres in size, with a narrow size distribution (2–3% of mean size) and tiny voids between the particles. Depending on the density and the pattern of the lattice of spheres, the lattice will diffract visible light in different and complex patterns. This diffraction, plus the interference patterns between the internal rays of light (reinforcing some wavelengths and cancelling others), creates the famous ‘play of colour’ in precious opal.
Smaller silica spheres (<150 nanometres) diffract the shorter wavelengths – blue and blue–green being the most common. The wavelengths diffracted then increase with the increasing size of the particles, with the larger spheres (>250 nanometres) giving the oranges and reds, as well as increasing the brightness of the diffracted light via the greater spacing between the spheres, the rarity of which together greatly increase the value of a precious opal. As noted by the Australian Museum: ‘Small dislocations and irregularities in the lattice of spheres then create bounded areas of colour that change with the angle of incidence of the light. Colour patterns that shift across the stone lend each opal its unique character’.
‘Common’ opal, known as ‘potch’ to miners, is chemically identical, but the silicon spheres are too small, more widely distributed in size, and/or too tightly spaced or disordered. It may be found in a wide range of colours, from white, through brown and green, to black. And while precious opal will fluoresce under UV light, common opal will not.
The most precious opals of all – black opals – are found only within a 70 kilometre radius of Lightning Ridge in north-western New South Wales. In these opals, the precious opal is typically only a very thin colour bar (often <1 millimetre) over dark/black potch, which better reflects the light through the precious layer, giving better clarity and pattern.
The waters of hydration of the silica spheres (SiO2.xH2O, where x can represent 2–20% but is more typically 6–10%) make opal vulnerable, particularly to rapid drying, exposure to sudden, intense light or intense vibration (such as during cutting and polishing). Any of these can cause unpredictable ‘crazing’ of the stone. Professional opal cutters often keep their uncut opal immersed in water while not ‘working’ the stone, and then only have it out of the water for just minutes at a time during cutting and polishing. Opal is also vulnerable to various chemicals, so care is needed when cleaning opal jewellery, but if a cut opal is carefully dried over a period of months (or even years), it can help stabilise the jewel to some extent.
So a fair bit is known about the structure and mineralogy of opal, but the mechanisms for formation of precious opal remain obstinately opaque. Across three major theories of formation, the processes range from chemical to bacterial, taking anywhere from a relatively small number of years to hundreds of thousands of years.
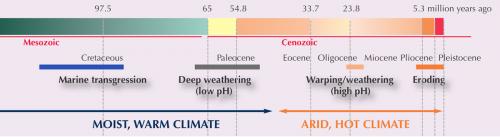
Let’s start with the agreed principles: Most of the world’s opal comes from a region of Australia that was, 122 to 91 million years ago, covered by a vast shallow epicontinental sea and was located much closer to the South Pole. The sediments in this sea were deposited from weathering of volcanic rocks, and were rich in organics from Cretaceous period bacteria, flora and fauna.
By the end of the Cretaceous, the region had moved considerably north, and what is now Central Australia probably looked something like today’s Amazon Basin. From about 40 million years ago, the climate became considerably more arid, and groundwater became more alkaline. Tectonic action continued lateral and vertical migration, and the climate changed. Any opal subject to surface weathering would have been destroyed.
The key question is ‘When did precious opal form’? Are precious opals contemporaneous with their ancient host rock, or did they form more recently? Does the apparent younger age of precious opal reflect new formation, or just ‘preservation bias’ from the erosion of older stone?
The weathering model is based on the observation of areas (such as Lightning Ridge) where sandstone overlays clay (often termed ‘opal dirt’ by miners). Opals are typically found in areas where the sandstone layer is thicker, but also shows faults or joints. It is thought that water infiltration during weathering carries dissolved silica or silica gels (from the sandstone) down to the clay layer, where it is trapped and precipitates. The process would be favoured by the presence of fresh pyrite, which forms sulfuric acid during weathering, and would thus promote the formation of silica gels.
However, the rarity of opals suggests greater complexity. In processes not well understood, the weathering model (as described by the NSW Department of Industry, Resources and Energy; bit.ly/1tDZECx) may involve such other factors as a change from an alkaline to an acidic environment; the presence of Al, Fe or Mg oxides; and the presence of NaCl or NaSO4.
As an alternative, the syntectonic model proposes opal formation as a result of geological activity. Concentrated, hot (>100°C) mineral-bearing water rising under pressure along fault lines or breccia pipes infiltrates sandstone and claystone layers that have been deformed by tectonic pressures. As the water dissipates into areas of lower pressure and temperature, opal is deposited as veins. This opal would form relatively quickly, and would not be contemporaneous with the rock strata in which it is found.
It is interesting to note that, in a New Zealand pilot plant in 2000, silica spheres of sufficient chemical purity were formed at 10–70 nanometres by simply cooling saline geothermal water (saturated with silica at ~500 mg/L and containing 1.5 g/L Cl, at pH 8.7) (bit.ly/1PBuOie).
Lastly, the microbe model points to the fact that the claystone rock that is commonly associated with opal contains large quantities of extremely fine, fossilised organic matter, particularly aerobic bacteria.
This model would have the opal formation as being contemporaneous with the formation of the host rock, and most abundant in the presence of the organic clay (smectite). It suggests that the Cretaceous sediments (97 to 60 million years ago) were abundant in organic matter, acting as a microbial habitat. Microbes feeding on these organics would have generated waste acids and enzymes, causing chemical weathering of clay minerals and feldspars (K/Na/Ca aluminosilicates) in the surrounding rocks and providing the source materials for opal formation.
A fairly heated 2014 exchange in the Australian Journal of Earth Sciences (doi: 10.1080/08120099.2013.870093) gives a pretty good indication of the ongoing discussion. The CSIRO argued for exclusively Cenozoic-era (65–2.9 million years ago) formation of precious opals, against a counter-argument for both Cenozoic-era and Cretaceous-period formation. Interestingly, the opalised fossil record is actually of assistance with the development of these theories. The Earthbyte Group (University of Sydney) points to the abundance of pre-Cenozoic precious opal fossils – plants, freshwater species, plesiosaurs, pterosaurs, dinosaurs, birds and mammals (noting that mammals first appeared in the fossil record around 150 million years ago, but became abundant in the Cenozoic era).
A 2011 paper from NSW Resources & Energy (bit.ly/1tvPCmg) reported abundant (up to 107–108/cm3) microbial fossils at 2–5 µm (ranging up to >100 µm) in both potch and host rock from Lightning Ridge. These actinomycetes and myxobacteria required a nutrient-rich and near-surface aerobic environment, with temperatures <35°C and pH ~7. The authors argued for potch opal formation over weeks or months, contemporaneous with the sediments in which they are found, and point to the excellent preservation of organic structures in the opalised fossil record. If the period of formation was slow, the authors argue, the organic structures would decompose and be destroyed.
The solubility of silica is very low in low-temperature water. During the early Cretaceous period, Lightning Ridge had a cold climate – even though there was a warmer global average (about 6°C) at the time, Lightning Ridge was closer to the pole than it is now. This is less than favourable for aerobic bacteria, but the authors argue that this would have generated higher amounts of organic acid, accelerating the bioweathering of the host environment by the now commonly understood processes and producing silica hydrosol (an aqueous suspension of colloidal silica spheres), in turn to produce opal potch. However, bacterial fossils, while common in potch, were notably absent from precious opal.
Collectively, the data I have read suggests that potch is formed pretty readily, and perhaps semicontinuously over geological timescales. It probably forms rapidly under the right conditions, and in doing so is able to preserve the structures of organic specimens including bacteria and wood, where the degradation of the original leaves a void for mineral formation. However, formation of these silicaceous deposits is too swift to be highly ordered or regularly sized, as required for precious opal.
The formation of precious opal must surely require a slower, more regular process to achieve order and regularity in the silica spheres. The detail preserved in fossil specimens that have undergone diagenesis (effectively mineral replacement of biological apatite with quartz, opal or calcite) requires tightly matched reactions with very little physical spacing at the reaction front as the mineralising water moves through the rock, and this suggests slow infiltration.
However, there remains an apparent mismatch between the limited radiodating of opal specimens primarily to the Cenozoic era and the apparent limitation of precious opal fossils to Cretaceous species. And there remains extensive ongoing argument as to the interpretation of the geological data on the timing of occurrence of precious opal versus the host rock.
Clearly, this is an area ripe for ongoing research. In the meantime, we can all take pleasure in the timeless beauty of this quintessentially Australian gemstone, and the magnificent creatures preserved in this manner.