The 2023 Nobel Prize in Physiology or Medicine was awarded jointly to Katalin Karikó (Professor at Szeged University, Hungary, and Adjunct Professor at Perelman School of Medicine at the University of Pennsylvania, US) and Drew Weissman (Roberts Family Professor in Vaccine Research and Director of the Penn Institute for RNA Innovations, US):
for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.
The discoveries of this year’s laureates were critical for developing effective mRNA vaccines against COVID-19 during the pandemic that began in early 2020. Through their groundbreaking findings, which have fundamentally changed our understanding of how mRNA interacts with our immune system, the laureates contributed to the unprecedented rate of vaccine development during one of the greatest threats to human health in modern times.
Producing whole virus-, protein- and vector-based vaccines requires large-scale cell culture. This resource-intensive process limits the possibilities for rapid vaccine production in response to outbreaks and pandemics. Therefore, researchers have long attempted to develop vaccine technologies independent of cell culture, but this proved challenging.
Genetic information encoded in DNA is transferred to messenger RNA (mRNA), which is used as a template for protein production. During the 1980s, efficient methods for producing mRNA without cell culture were introduced, called in vitro transcription. However, in vitro-transcribed mRNA was considered unstable and challenging to deliver, and in vitro-produced mRNA gave rise to inflammatory reactions.
These obstacles did not discourage Hungarian biochemist Karikó. During the early 1990s, when she was an assistant professor at the University of Pennsylvania, she remained true to her vision of realising mRNA as a therapeutic.
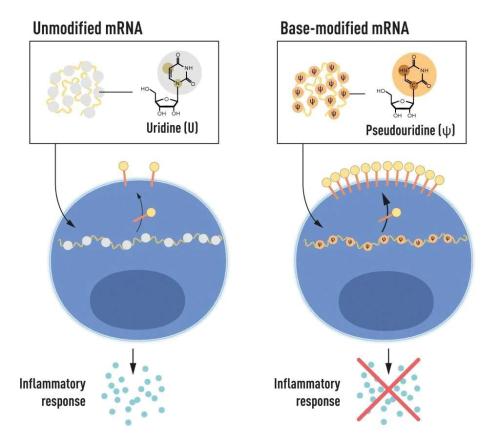
Karikó and colleague immunologist Drew Weissman noticed that dendritic cells recognise in vitro-transcribed mRNA as a foreign substance, which leads to their activation and the release of inflammatory signalling molecules. They wondered why the in vitro-transcribed mRNA was recognised as foreign while mRNA from mammalian cells did not give rise to the same reaction. Karikó and Weissman realised that some critical properties must distinguish the different types of mRNA.
Karikó and Weissman knew that bases in RNA from mammalian cells are frequently chemically modified, while in vitro-transcribed mRNA is not. They wondered if the absence of altered bases in the in vitro-transcribed RNA could explain the unwanted inflammatory reaction. To investigate this, they produced different variants of mRNA, each with unique chemical alterations in their bases, which they delivered to dendritic cells. The results were striking: the inflammatory response was almost abolished when base modifications were included in the mRNA. Karikó and Weissman immediately understood that their discovery had profound significance for using mRNA as therapy. These seminal results were published in 2005.
In further studies published in 2008 and 2010, Karikó and Weissman showed that the delivery of mRNA generated with base modifications markedly increased protein production compared to unmodified mRNA. The effect was due to the reduced activation of an enzyme that regulates protein production. Through their discoveries that base modifications both reduced inflammatory responses and increased protein production, Karikó and Weissman had eliminated critical obstacles on the way to clinical applications of mRNA.
Interest in mRNA technology began to pick up, and in 2010, several companies were working on developing the method. Vaccines against Zika virus and MERS-CoV were pursued; the latter is closely related to SARS-CoV-2. After the outbreak of the COVID-19 pandemic, two base-modified mRNA vaccines encoding the SARS-CoV-2 surface protein were developed at record speed. Protective effects of about 95% were reported, and both vaccines were approved as early as December 2020.
The impressive flexibility and speed with which mRNA vaccines can be developed pave the way for using the new platform also for vaccines against other infectious diseases.
In the future, the technology may also be used to deliver therapeutic proteins and treat some cancer types.
Several other vaccines against SARS-CoV-2, based on different methodologies, were also rapidly introduced, and together, more than 13 billion COVID-19 vaccine doses have been given globally.