On 15 September 2023, the US Food and Drug Administration (FDA) announced that momelotinib, an orally bioavailable JAK1/JAK2 kinase inhibitor (JAK1 and JAK2 being enzymes that catalyse the transfer of a phosphate from ATP to specific signalling proteins), was approved for the treatment of myelofibrosis for patients with anaemia. For these patients, the approval represented an important advance for the treatment of their disease. For GSK, the global pharmaceutical giant that filed the New Drug Application with the FDA, it meant the beginning of a marketing campaign for a drug they predict will be a blockbuster (meaning annual sales of more than US$1 billion). And for a small group of scientists in Melbourne, your author included, it meant that the more than 20-year odyssey to discover and develop the drug had finally come to a triumphant conclusion.
Momelotinib is only the second drug invented in Australia to be approved by the FDA for sale in the US – the world’s largest pharmaceutical market. Further, the drug’s primary targets – the kinases JAK1 and JAK2 – were discovered in Melbourne, and in addition the discovery team, in close collaboration with academic scientists from Monash University, were the first in the world to obtain crystal structures of both kinases. Taken together, these achievements represent a high-water mark for medical science and drug discovery in Australia.
Unfortunately, however, the drug development activities, which began in Melbourne, were predominantly carried out by companies overseas. The momelotinib story is therefore another example of Australian science not being supported locally through its development journey, and being sold to overseas entities before significant benefits from commercialisation could flow back to local investors and the Australian drug discovery and development ecosystem more broadly.
Discovery of the JAKs
The JAKs were discovered by Professor Andrew Wilks, a laboratory head at the now-defunct Melbourne laboratories of the Ludwig Institute for Cancer Research. Wilks was building on the seminal work in the Parkville precinct in haematopoiesis, spearheaded by luminaries such as Professor Don Metcalf, trying to unravel the processes that, from a small number of precursor cells, gave rise to the many cells in our blood. Wilks invented a process to identify intracellular enzymes in precursor cells essential for their differentiation and maturation, and during this work discovered two enzymes called Just Another kinase 1 and 2 (JAK1 and JAK2), later renamed after the two-faced Roman god of doorways, Janus, to reflect the homodimer formed by the kinase units (Molecular and Cellular Biology 1991, vol. 11(4), pp. 2057–65).
The birth of Cytopia
In the 1990s, it was essentially unheard of for academics to leave academia and start a company, but Wilks did just that, founding the company Cytopia off the back of his discovery of the JAKs in 1997. He licensed the seminal JAK intellectual property from the Ludwig Institute and, after a number of years trying to secure funding, was finally successful in securing investment from the ASX-listed ‘Pooled Development Fund’ Medica Holdings. Medica, headed by chemist Kevin Healey, at that time was funding drug discovery activities at two other Australian biotechs, Xenome and Alchemia. Cytopia started its early lab work in the Gaol Ward of St Vincent’s Hosptial in Fitzroy; not an auspicious beginning.
With funding in place, Wilks started building Cytopia’s capabilities in kinase drug discovery. While the primary focus was on the kinase JAK2, an extensive panel of other kinases with known roles in cancer and/or inflammatory disease biology was also included, including JAK3, FAK, cFMS, cMET and KDR, among others. The team’s initial focus was on establishing drug screening capabilities using both cell-free and cellular systems and, with these in hand, screening of small collections (up to 1000 compounds) of commercially available ‘kinase-targeted’ chemical libraries began. Hits in the cell-free screen against the JAK2 enzyme were seen early on, and at this time Wilks and his small group of biologists came to the realisation that they needed chemistry know-how to move things forward.
Drug discovery begins
I joined Cytopia at the beginning of 2001 after starting my medicinal chemistry career at Pfizer UK in the early 1990s. In Cytopia, and under Wilks’ leadership, it was clear a unique capability with excellent people had been established to undertake target-driven drug discovery. In addition to drug screening capability, Wilks had enlisted the skills of molecular modellers Herbert Treutlein and Jun Zeng, both previously of the Ludwig Institute, who were developing a unique and advanced virtual screening platform, well before machine learning and AI was de riguer in the industry. We opened chemistry labs at the Baker Institute, and began making and testing compounds designed from the initial hit compounds. Early work from this program focused on compounds with cell-killing activity against cancer cell lines, particularly prostate cancer cells, since this cancer had a documented JAK2 dependency. Optimisation of this series of compounds led to the discovery of the potent vascular disrupting agent CYT997 (lexibulin) that entered the clinic for treatment of solid tumours. While itself not a JAK2 inhibitor, this compound demonstrated the capability of the team to discover and develop compounds for clinical study.
JAK2 and myeloproliferative neoplasms
The JAK2 program gained momentum in 2005 after several scientific reports were published identifying an activating mutation in JAK2 as being the likely molecular cause for myeloproliferative neoplasms, a family of blood cancers in which over-production of specific cells in the blood leads to severe disease. Of these myeloproliferative neoplasms, the worst was idiopathic myelofibrosis, essentially a scarring of the bone marrow, for which there was no effective treatment and where patient median survival was approximately five years. In addition, these patients suffered from a suite of debilitating symptoms, including spleen enlargement, bone pain, night sweats, fever and chronic itchiness (pruritis), before ultimately succumbing to the disease.
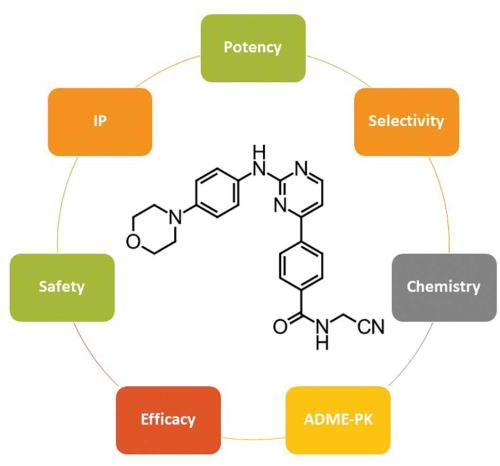
Our JAK2 drug discovery program was already well advanced by the time of these reports, so we rapidly refocused attention to completing the optimisation work. The chemists involved in this work included Andrew Donohue, Michelle Farrugia, John Feutrill and Thao Nguyen, along with many others. Working with our molecular modellers along with talented structural biologists at Monash University (Professor Jamie Rossjohn, Isabel Lucet and Onisha Patel), we built a deep understanding of the ATP-binding site of JAK2 – the pocket in which our drugs bind to out-compete ATP and thereby stop the enzyme working. With these insights, we designed a series of potent JAK inhibitors and ran these through a battery of enzyme and cellular assays, as well as testing for selectivity, assessing pharmacokinetics and adsorption, distribution, metabolism and excretion (ADME), and undertaking preliminary safety profiling. From this work, CYT387 (momelotinib) emerged as the most promising compound: not necessarily the most potent or active in individual assays, but the best across all assays and studies. Importantly, CYT387 was active in cellular assays using cells derived from myelofibrosis patients, and in a genetic mouse model of myeloproliferative neoplasms. Armed with this combined dataset, we chose the compound as a development candidate and patented it in 2007. Formal preclinical studies began in 2008 under the leadership of Gregg Smith and in 2009 the first clinical trial began at the Mayo Clinic in the US, under the guidance of Professor Ayalew Tefferi, a world leader in myeloproliferative neoplasms.
Momelotinib leaves the country
In 2009, the global financial crisis was in full swing and Cytopia was unable to continue to fund the development programs underway. First the research team were let go, and within a year the company was sold to Canadian drug developer YM BioSciences for US$14 million.
The team at YM quickly realised the quality of the JAK2 program and continued to fund its development, generating exciting phase 2 data that resulted in the company’s acquisition by Gilead BioSciences in 2012. Gilead purchased YM, solely for the JAK2 program and momelotinib in particular, for US$510 million. Clearly, two years and solid data can add a lot of value to a drug program!
Gilead initiated a phase 3 study, called the SIMPLIFY study, comparing momelotinib to the comparator JAK2 inhibitor ruxolitinib, a drug developed contemporaneously by the US biotech Incyte. The trial headline data reported in 2016, that there was little difference in the drugs with respect to spleen size reduction, was not sufficient in Gilead’s eyes to warrant further development and they shelved the program. This was in spite of data showing that momelotinib-treated patients with disease-related anaemia had very profound responses, showing significantly improved iron levels and better patient outcomes. This could have been the end of momelotinib had it not been for the team from YM, now working for a new US biotech called Sierra Oncology. They negotiated a deal to purchase the compound from Gilead and planned a new phase 3 trial, working closely with the US FDA. That trial, the MOMENTUM trial, read out in 2021 and clearly demonstrated the efficacy of the drug in terms of spleen reduction, improvement in constitutional symptoms and importantly reversal of anaemia.
In April 2022, GSK purchased Sierra Oncology for US$1.9 billion, ostensibly for momelotinib, and then went about filing the New Drug Application with the FDA. The approval in September 2023 represents the final step in this long journey. Now to observe the drug’s fortunes as a medicine approved ‘to treat adults with certain types of myelofibrosis (MF) who have anemia’.