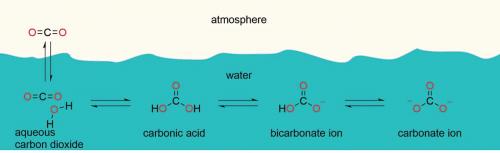
The control of the oceans’ pH is dominated by that most important buffer bicarbonate, which also controls the pH of your blood and saliva.
pH in the oceans
The average pH of unpolluted ocean surfaces is about 8.1, having decreased from an estimated 8.2 in pre-industrial times.
A drop in pH of 0.1 might seem trivial but a logarithmic scale such as that used to represent pH distorts by compressing large numbers and expanding small ones. It is needed for scales that cover a range of magnitudes (such as the decibel, lumen, and Richter scales), but for chemical calculations, absolute concentrations are required.
If a decrease in pH of 0.1 in the ocean is reported as a 26% decrease, then lemon juice (pH 2), is 30 000% more acidic than soda water (pH 4.5).
When seawater begins to evaporate at the edge of drying ocean pools, the white stuff that you see is not (as most believe) common salt (the overwhelmingly major solute of oceans); first, it is limestone (when roughly 50% of the water has evaporated) and then gypsum (when 80–90% of the water has evaporated), and only then do lovely clear cubic crystals of common salt form (when 85–95% of the water has evaporated).
Salt from seawater is traditionally purified by evaporating the water from a succession of pumped or gravity-fed pools of increasing salinity to first separate out the less desirable, less soluble salts (see Maxwell I.A., Chem. Aust. December 2014–January 2015, pp. 36–37).
More CO2 in the atmosphere
Increasing atmospheric CO2 from fossil fuels means more CO2 will eventually dissolve in the rivers and oceans. ‘Increased acidity’ suggests a pH less than 7, so ‘reduced alkalinity’ is clearer.
Unlike other acids, CO2 has a dual role; it also supplies a common ion, namely carbonate.
Because of a very high concentration of spectator ions such as sodium in seawater, calcium carbonate is at least 60 times more soluble than it is in fresh water.
Ocean equilibria
The following diagrams display prevalence of species and serial equilibria in seawater.
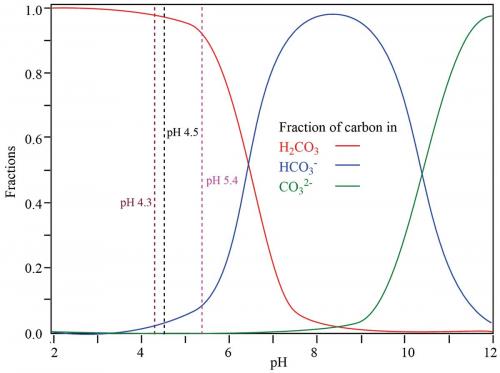
What is a common ion? In the top diagram, you can see that adding CO2 shifts the equilibrium to the right but adding carbonic acid shifts the equilibrium to the left. These opposing actions roughly balance until the pH eventually drops to about 7 where bicarbonate buffering fails significantly (see graph).
Simple activity calculations predict that the solubility product Ksp(CaCO3) is 5.4 × 10–7 in seawater at pH 8.1, but is 8.7 × 10–9 in fresh water – about 60 times lower. Kinetic control (e.g. rates of precipitation and dissolution), rather than thermodynamic control, is determinant. Tough stuff.
Many other factors, including local pollutants, can play a large (sometimes unintentional) role.
Oysters, corals and carbonate
The chemistry of the oceans is very complicated; biochemical processes involving enzyme-mediated reactions are even more complicated. Organisms adapt differently to changing environmental stress; there are winners and losers from Darwinian pressure (bit.ly/3iVNbq6).
In some parts of the ocean, natural pH fluctuates substantially. Some corals can protect themselves from fluctuating pH because they control their internal pH environment, so adding excess CO2 to seawater around these corals has no effect on their survival. A good example is the corals on Heron Island in the Great Barrier Reef (go.nature.com/3jc0v8D). Oysters build their carbonate–protein-layered shells (and pearls) biochemically, from the inside out, while controlling their internal pH at 6.8.
Why would ocean organisms choose the hard-to-get carbonate rather than the dominant highly soluble bicarbonate as their source? This is still being investigated, but it has been reported that some control their internal pH independent of the oceans, as well as utilising both bicarbonate and carbonate (bit.ly/37eEQqR).
Ocean warming transfers CO2 from the oceans to the atmosphere, which helps the former but harms the latter. Increased temperature reduces the solubility of solid calcium carbonate. Increased pressure, as with increasing ocean depth, increases its solubility.
The severe negative consequences of increased CO2 in the atmosphere aligns with centuries-old accepted physical chemical principles.
But a hyped threat from a pH drop in the Great Barrier Reef does not make any chemical sense.
Sit, as I have, in a limestone cave and watch the pH 7.6 bicarbonate-buffered water flow down and drip, whereby the formations slowly grow, not shrink.
In the words of US journalist Henry Mencken (1880–1956): ‘For any complicated problem there is a simple solution that is wrong’. We ignore basic STEM topics … pH, equilibria, buffers, activity and particularly biology and biochemistry ... at our peril.