While life is being overrun by tiny, spiked packets of biochemicals that are causing COVID-19, I’ve been watching in amazement the microbiologists, virologists, epidemiologists and infectious disease researchers explaining the complex biochemistry of this virus. To appreciate how this science reached such an advanced level, a look at the person who laid its foundation – Louis Pasteur – is a great place to start.
The Pasteur family origins were in various small villages in the Jura mountains, which straddle the present-day French–Swiss border. The Pasteurs were agricultural workers dependent on local priories, one family of which in the1600s moved to the fertile plains below, to the town of Dôle where Louis Pasteur was born in 1822.
Louis’ family moved to nearby Arbois in 1827, and it is here that Louis grew up, with his parents teaching their children the values of family loyalty, respect for hard work and financial security. At primary school, Louis readily led reading groups, as was fashionable at the time, although he only achieved moderate grades. His great love was pastel drawing and painting, and he completed a sizeable portfolio of works, using a wide variety of artistic techniques. It has been commented that Louis’ paintings show the intensity and attention to detail that would serve him well in his scientific career.
During Louis’ youth, influential educators and philosophers frequented the Pasteur family home and they saw something in the young man’s determination and demeanour. They encouraged him to apply for admission to the École Normale Supérieure in Paris, being originally established during the French Revolution as a training school for professors to teach the new ideas of the Enlightenment. Louis, at the age of 20, completed the entrance examinations for the École and was admitted, ranked 16th. Displaying his urge for perfection, Louis was not content with his rank and refused the École’s offer. He waited another year, during which he further prepared for the entrance exams and, in 1843, his second attempt resulted in a higher ranking of fifth. He was happy to accept this and entered the École. This determination for perfection was another hallmark that Louis would bring to his scientific work.
Tartrates and optical activity
For his doctorate, Louis chose to study crystallography, an area of great interest at the time, with a number of unusual optical phenomena being observed in mineral crystals. But Louis was interested in a material a bit closer to his heart – the organic compound tartaric acid – a by-product of wine fermentation. This was found in thick, crusty deposits in wine barrels, familiar to him from the vineyards and wineries of his Jura plains boyhood. He had read extensively about the compound, as it had been known for some time that it existed in two forms: a large crystal form called tartaric acid and, occasionally found with it as a smaller needle form, paratartaric acid (now called DL-racemic acid – from the Latin racemus meaning ‘grape’). What had puzzled chemists of the time was that, despite the two forms being chemically identical, a solution of the tartaric acid salt (i.e. the tartrate) rotated a plane of polarised light, while an equivalent solution of the paratartaric acid salt (paratartrate) was inactive.
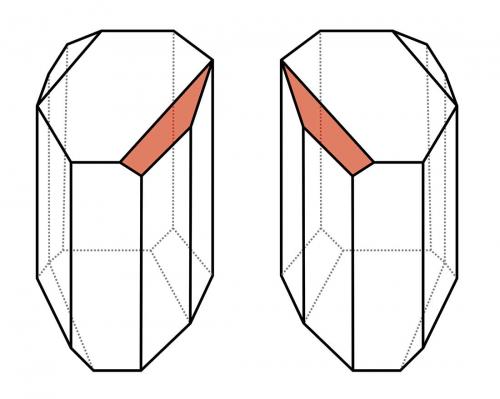
Louis’ approach was to look at the crystals of the acids under a microscope. This was not a new approach, but Louis saw what others had missed: the tartrate salts were hemihedral in form, having little faces on one half of the edges. Louis’ keen artistic sketching eye picked up that the crystals of paratartrate salts were of the same shape but fundamentally different, being mirror images of themselves. The tartrate salt crystals, however, were all of one mirror image form or handedness. Louis then displayed his experimental brilliance by separating the two types of crystals in the optically inactive paratartrate salt, using tweezers under his microscope, and then making them up in separate solutions. As he expected, one solution rotated the plane of polarised light to the right, the other to the left. He had prepared solutions of tartrate salt and its optical isomers (enantiomers). Mixing the solutions once again resulted in an optically inactive mixture.
The reasons that some molecules have the property of chirality, forming optical isomers that rotate polarised light differently, would not be understood until much later, but Louis’ discovery hastened the search for this understanding. Louis was also fortunate that he was studying mixtures of optical isomers that display the rare property of conglomerate crystallisation, forming mirror image crystals reflecting their parent compound’s structure.
Unique experiments with fungus
With this remarkable discovery, Louis’ reputation began its crescendo and he was able to secure a position as Professor of Chemistry at the University of Strasbourg in 1848. There he met Marie Laurent, a daughter of one of the university officials and two weeks later, being quite smitten by her, wrote a letter to Marie’s father asking for her hand in marriage. Marie agreed and they were married in 1849 with Marie becoming a constant companion and assistant to Louis, always showing interest in his work and encouraging him. Later in life she wrote to their five children, ‘Your father is absorbed in his thoughts, talks little, sleeps little, rises at dawn, and in one word continues the life I began with him this day thirty-five years ago’.
At Strasbourg, the thrust of research work was still tartrate salts and, after pursuing some dead ends, he devised and completed another one of his decisive, brilliant experiments. He had observed, as others had in the university, that a certain fungus would grow in calcium tartrate solutions in warmer weather, spoiling them, which meant they had to be disposed of and replaced with fresh solutions. Louis wondered how the fungus would metabolise the mixture of optical isomers he’d observed previously; would it metabolise both equally? To address this, Louis grew the fungus in an optically inactive paratartrate solution (a mixture of both optically active isomers) and took progressive samples of the medium during the fungus’ growth, measuring its optical rotation. To his great excitement, he found the solution became progressively more optically active, rotating polarised light to the left. He produced crystals from the solutions and once again, with his tweezers under a microscope and his sharp eye, separated the two mirror image crystals and measured their relative amounts. He confirmed that the fungus was preferentially removing the right light-rotating isomer from solution. It was already known that plant-derived compounds were optically active, but the same laboratory-synthesised compounds were not. Louis’ observations advanced the understanding of this phenomena markedly. He made much of this discovery, perhaps not fully appreciated by his contemporaries, claiming that it was key to understanding the origin of life. The significance of the discovery is increasing as chirality and stereochemistry are being used to search for life in extreme and astronomical environments. It appears to be unique to Louis, with no other researchers performing similar experiments leading to this significant finding.
Ferments, flasks and filters
Louis’ research in Strasbourg had attracted more fame and monetary prizes, the latter spent mainly on laboratory equipment. Despite this, his laboratory facilities were still extremely limited so in 1854, at the age of 32, he accepted an appointment to the chair of chemistry and the dean of sciences at the newly reorganised University of Lille in the north of France.
At Lille, Louis became more involved in wine production problems, probably from his initial contacts he had made chasing various tartrate samples. Some of his ‘pure science’ colleagues did not approve of this work, with Louis’ riposte being ‘There are not two different kinds of science; there is science and there are the applications of science’. The issue du jour among the local vignerons was inexplicable wine spoilage during production. The biochemical process of what we now call fermentation was not understood at the time. The current theory was spontaneous generation – the observed transformations thought to be the result of the materials in the medium somehow rearranging themselves to form new products. Louis once again decided to tackle the problem by looking at it under his microscope, which he did while holidaying at his country home at Arbois (where he had a fully equipped laboratory). There, he collected many grape juice samples at various stages of fermentation and made his characteristically detailed observations. In unspoiled samples, he saw ‘particles’ that others had already noted, but once again his keen eye observed more than others: small ‘buds’ seemed to form on these particles, with these buds eventually separating, growing and repeating the budding process. His mind started to tick – he had also observed that the grape juices were optically active, isolating an optically active component amyl alcohol. Were these particles the yeast? Was it a living organism, like his fungus, preferring one optical form and responsible for the conversion of sugar to alcohol? He furthermore observed that the ‘particles’ in spoilt wines were smaller and of different shape. Were these competing yeast-like organisms that had the disrupted the normal yeasts?
And what of the souring of milk where sugar is converted to the unpleasant-tasting lactic acid – was this also due to these small living particles or ‘ferments’? Louis discovered that these fermentation processes could be slowed down or sped up by adding various agents that changed such properties as acidity and temperature. He also developed agents, which he called ‘antiseptics’, that would kill these living particles. These techniques were important predecessors to his later virus work.
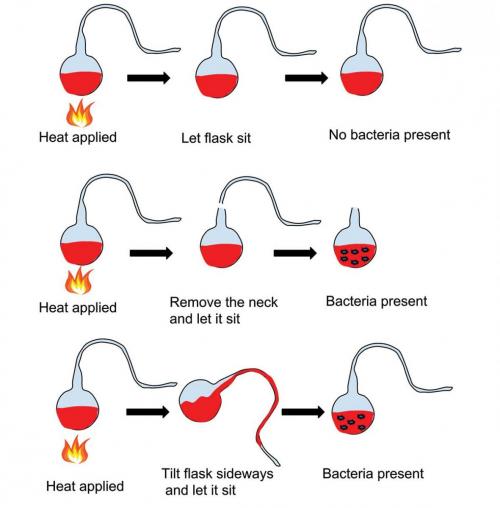
In 1857, Louis returned to the school he had graduated from in Paris, the Ecolé Normale Supérieure, as its assistant director, once again hoping that his rise through the administrative ranks would give him access to bigger laboratory facilities. He continued his fermentation studies, where he tried to determine whether these processes were the result of living organisms. To achieve this, Louis performed a set of characteristically brilliant experiments using a specially designed piece of glassware – the swan-necked flask. He began to make the flask by placing some fermentable fluid into a boiling flask after which he drew the flask’s neck upwards into a thin tube, then further caressed the tube into an S-bend at right angles to the flask’s neck. He then boiled the contents of the flask, the generated steam flushing the ambient air from it. Cooling resulted in the external air being drawn back into the flask through the contorted S-bend. Various particles in the air containing yeast and other microorganisms were trapped during their journey in the lower S of the bend, resulting in the flask’s liquid remaining clear and unaltered. Tipping the flask so the liquid contacted the trapped material in the S-bend and then re-inverting resulted, after a short while, in fermentation. Some of these flasks are on display at the Pasteur Institute in Paris, with their contents still clear and unaltered more than 100 years after their preparation. A remnant of the swan-necked flask is the pasteur pipette, present in many of today's laboratories.
Louis did not rest; he followed up these experiments by filtering air through gun cotton and microscopically observing the entrained microorganisms. He then grew and ‘cultured’ his yeasts in novel synthetic mixtures of nutrients and noticed that some microorganisms did not require oxygen but survived in oxygen-free, anaerobic environments. He had founded the science of microbiology.
Setting his sights on French agriculture, Pasteur introduced various hygiene and heating treatments to promote the desired microorganisms, and reduced the spoilage of wine, beer and milk. The heat treatment of raw milk to increase its shelf life still bears his name – pasteurisation.
During these studies, the sanctity of Louis’ laboratory, which he always insisted on, was somewhat challenged: the tasting of beer from new production techniques, punctuating the usual silence with the clinking of glasses and gentle laughter. On one occasion, when Louis was enthusiastically describing the technical details about beer production and microbiology to one of his assistants, the assistant was heard to say ‘Mr Pasteur, you do realise the purpose of making beer is to drink it!’
Louis had also linked microorganisms with putrefaction and now began to promote the concept of them also causing human disease. Based on Louis’ work, Scottish surgeon Joseph Lister put in place meticulous precautions to prevent microorganism contamination during his surgical procedures, thus reducing the surgical mortality rate dramatically.
Silkworms, cholera and anthrax
Following Louis’ swan-necked flask work, fate was to give him a chance to study disease more closely – not in humans, but in silkworms. Learning that a mysterious disease had afflicted France’s silkworms, causing major economic hardship, he decided to investigate, completing the work from 1865 to 1870 in a makeshift laboratory in a house in Alés, southern France. Louis worked assiduously, carefully studying every step of the silkworm’s life cycle with his trusty microscope, discovering that the silkworms were not spinning their valuable silken cocoons because of two diseases – pébrine and flacherie (now known to be caused by a protozoan parasite and a virus respectively). From his observations, he was also able to determine which silkworms were infected with the diseases and trained silkworm farmers to remove infected silkworms from their stock, gradually purging the disease. This was the first success of laboratory science in control of infectious disease.
Louis moved up a gear to study a then common disease causing the death of farmed animals – anthrax. Louis’ study of this disease concentrated on the developing immunity in uninfected animals. He had also been studying chicken cholera, caused by the bacterium Pasteurella, and had noticed that cultures of chicken cholera left on the Arbois laboratory shelf over summer no longer killed chickens. Furthermore, when these chickens were injected with the normal cholera bacteria, they did not die. Preparing this attenuated culture at elevated temperature, Louis found it could routinely prevent cholera in chickens that had been injected with it. Using similar attenuation techniques, he attenuated the sheep anthrax bacteria, which was decimating flocks throughout France, and, in a spectacular field trial in 1882, successfully demonstrated the viability of his vaccine.
The ultimate challenge – human rabies
Louis applied the techniques he had developed with chicken cholera and anthrax to rabies. The decision to study rabies is seen by historians as odd because the disease caused a comparatively low number of deaths in France at the time. However, when he was a boy, Louis had witnessed a rabid wolf terrorising the inhabitants of Arbois, biting people and animals alike in a frenzied attack. He also witnessed attempts to save the bitten casualties by cauterising the wounds with red-hot blacksmith irons, and the suffering of those who contracted the disease. Rabies is caused by a virus and is usually transmitted in people through contact with saliva with broken skin, typically via a bite from a rabid mammal. The virus travels through the central nervous system and reaches the brain, after which symptoms appear. This incubation period is typically several weeks but is less if the bite is closer to the brain. Severe neurological symptoms develop and, even to this day, if symptoms progress to this stage the disease usually results in death.
Louis began his work by looking at rabies-infected material under a microscope and could find no causative agent. He carried on regardless, suspecting that it was a smaller microorganism than bacteria, and developed entirely new methods to handle, culture and attenuate it. Louis’ trusty laboratory assistant of many years, Emile Roux, devised how to study the length of survival of the virus in the spinal cord of rabbits, using a specially designed bottle with two openings. Louis adapted Emile’s techniques and suspended rabbit spinal cords containing the virus in the bottles with some desiccant, producing a non-virulent form of the virus. Courses of emulsions made from these attenuated spinal cords were inoculated into dogs and found to provide immunity towards the disease.
The next step was possible human trials, which Louis did not look forward to, knowing full well the horrors that an unsuccessful trial could bring in terms of possibly causing untreatable rabies in the patient. Opposition also came from abroad, from the eminent microbiologist Robert Koch, and from within, with Emile Roux stating that the potential vaccine was far too dangerous and that he would not have anything to do with any human trial. But Louis’ hand, and his heart, was forced by fate, when in 1885 two doctors brought to him a young boy, Joseph Meister, who had been viciously bitten by a rabid dog. The two doctors knew of Louis’ work and were convinced that since rabies had a long incubation time, a course of Louis’ vaccine during this would cause immunity in the patient. Louis was not a trained medical doctor and thus could not administer the vaccine; the two doctors said that they would take full responsibility for the boy’s fate. Louis must have known that the boy’s life, his personal reputation and any further chance of using the vaccine was at stake. He conceded, and after 12 successive inoculations of increasingly stronger attenuated virus, the young boy did not develop rabies symptoms and was allowed to go home. A second success followed, involving a young shepherd boy, and after 15 months 2490 people had received the vaccine.
The indefatigable Louis completed much more research, but the development of the rabies vaccine was his last major achievement. In his lifetime, he received many awards and adulations, and he lobbied the government to set up a purpose-built research institute, which to this day bears his name. His application of theoretical work to solve major agricultural and societal health problems is a showcase for the power of scientific research. Louis had a stroke in 1894, from which he did not recover. He died on 28 September 1895, near Paris. He was given a state funeral and his remains are interred in a vault of the Pasteur Institute in Paris.