This story begins with a close friend of mine, who had been unwell for some time. He became very weak, and his family insisted on taking him to hospital. A day of testing culminated in the diagnosis of Addison’s disease. My friend was on the brink of a medical crisis, and his family probably saved his life.
I would like to share with you what I have learned and now appreciate about Addison’s disease and the powerful chemistry of the endocrine system.
Most medical sources classify Addison’s disease as a rare condition, affecting 1 in 100 000 people, affecting males and females equally but more prevalent in the 30–50-year age group. It can go undetected for some time and is often suspected in people who fail to recover from minor illnesses easily or, more dramatically, have an adrenal (Addisonian) crisis (see below). Debilitating symptoms of the disease include nausea, fatigue, anorexia, anaemia, vomiting and low blood pressure.
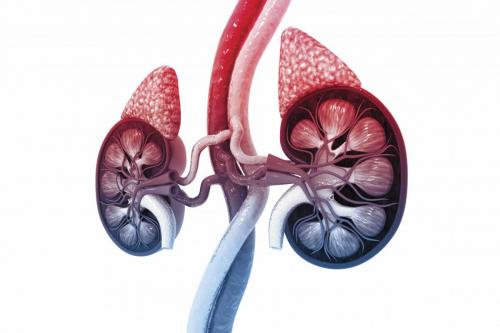
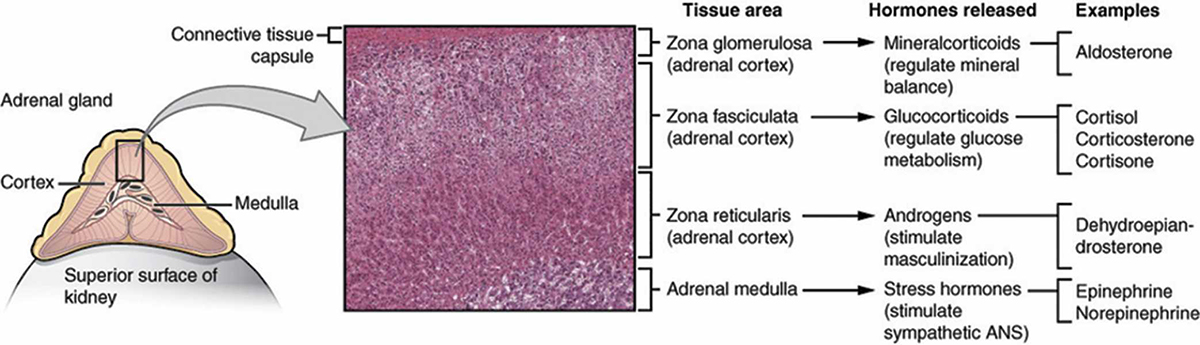
Addison’s disease is caused by the altered function or deterioration of the adrenal glands. These two small glands – one ‘capping’ the top of each kidney and weighing 1.5–2.5 grams – are an important component of the body’s endocrine system, which also includes the hypothalamus, pituitary, thyroid, parathyroid, pancreas and ovaries/testes.
The endocrine system regulates cellular and organ function to maintain a constant internal environment, which is critical for the body to operate.
To achieve this environment, the endocrine system regulates and coordinates, among other things:
- sodium and water to control blood volume and pressure
- calcium and phosphate to maintain cell membrane integrity and intracellular signalling
- energy balance and optimisation of fuel utilisation
- haemodynamic (blood flow) and metabolic responses
- reproduction, development, growth and ageing.
The endocrine system affects distant target organs by secreting messenger molecules (hormones) directly into the bloodstream. Hormones have a wide variety of structures, in the form of proteins/peptides, steroids or amino acid derivatives.
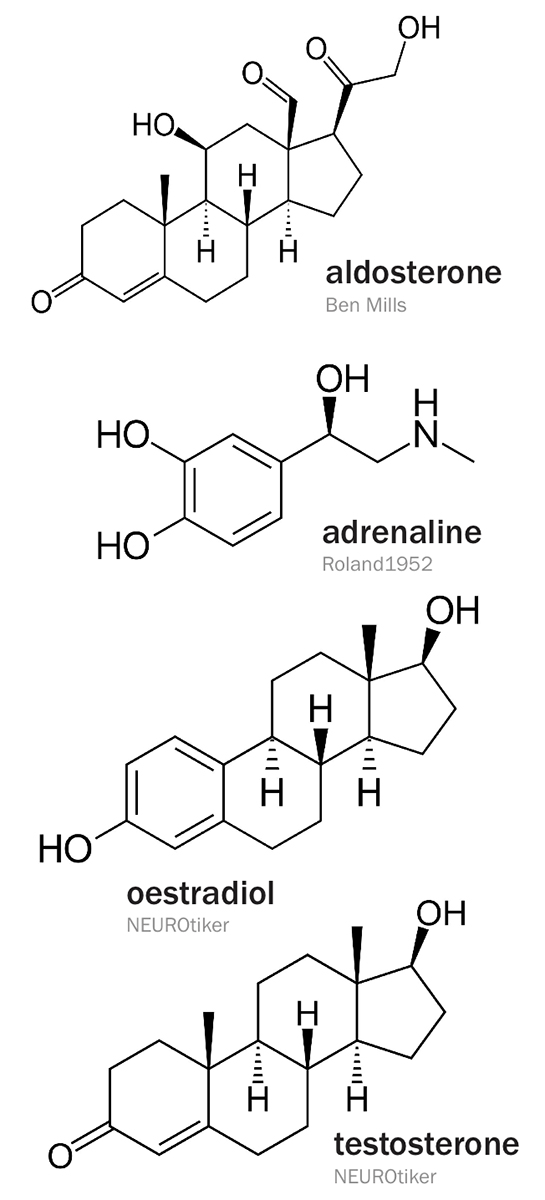
Several diseases are related to the function of the adrenal glands. Addison’s disease, or adrenal insufficiency, is a partial or complete failure of the adrenal glands to produce cortisol and, in some cases, aldosterone. Primary adrenal insufficiency can be caused when the adrenal glands are damaged by autoimmune disease, tuberculosis, infection or cancer. Secondary adrenal insufficiency can be caused by insufficient ACTH production by the pituitary gland, resulting in adrenal gland atrophication.
Cortisol is important for muscle function (including cardiovascular function), and a deficiency can result in muscle weakness. The lack of cortisol causes food to move more slowly through the gastrointestinal system, and reduces iron and vitamin B12 absorption. Appetite decreases, which, coupled with gastrointestinal dysfunction, often results in weight loss. Cortisol is important in glucose metabolism, so reduced levels disrupt several metabolic pathways, and blood glucose can become dangerously low (hypoglycaemia).
In primary adrenal insufficiency, both cortisol and aldosterone production are affected. This can increase potassium (hyperkalaemia) and decrease sodium (hyponatremia) in the blood. Coupled with the associated dehydration, and left untreated, these changes can have serious health consequences.
An Addisonian crisis may present as sudden loss of strength, pain in the lower back, abdomen or legs, vomiting and diarrhoea, low blood pressure and fainting. If untreated, it can lead to life-threatening shock, seizures and coma. In these cases, administering water too quickly may be harmful: ‘water poisoning’ can occur because the loss of cortisol impairs the ability of the body to expel water. It is understandable that people with Addison’s disease, faced with so many health issues, are prone to mood changes and depression.
When adrenal cortex cortisol production is compromised, the pituitary gland, as part of a feedback loop, produces more ACTH to compensate. Another effect of these elevated levels is skin darkening because the body recognises the ACTH as melanocyte-stimulating hormone (MSH). This is because a 13-member amino acid sequence in ACTH's structure corresponds to that of MSH.
Melanocytes are melanin-producing cells and are the reason for the colour of moles, freckles and suntans. They have an important role in protecting the skin from ultraviolet radiation. Very high levels of ACTH in Addison’s disease act like MSH, increasing melanin deposition and skin pigmentation. In fact, early in the disease people may actually be pleased because they lose weight and have a nice suntan, without any effort or the risks associated with excessive sun exposure. However, this is not a good way to get a tan because patients eventually become ill and may die if medical treatment is not sought.
The good news is that Addison’s disease can be treated effectively and inexpensively. Cortisol was isolated in 1949 and it was deemed a wonder drug, although we know now that too much can be harmful.
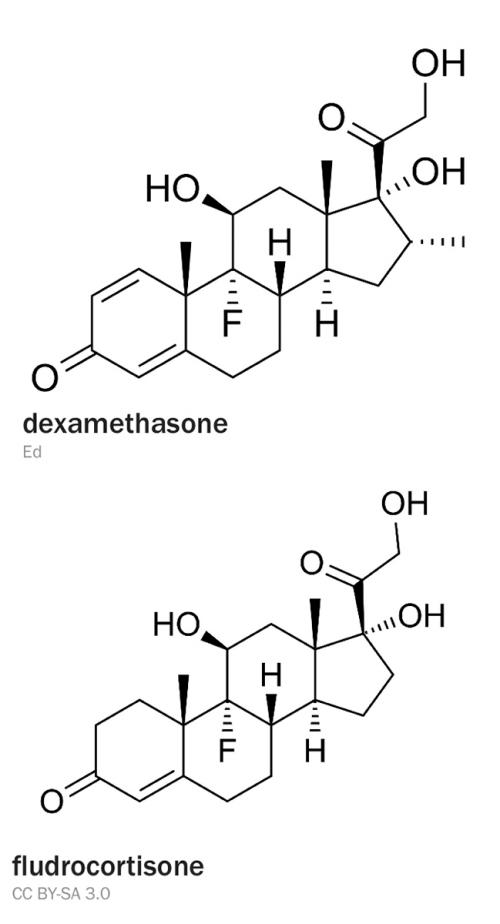
Either cortisol or cortisone can be taken orally (cortisone is biologically inactive and converted by the body to cortisol). Synthetic alternatives such as dexamethasone can be used, but since such compounds only have glucocorticoid activity, a synthetic mineralocorticoid such as fludrocortisone must also be taken for secondary insufficiency, where aldosterone production has been compromised. People with Addison’s disease must increase their glucocorticoid dose accordingly (but not the mineralocorticoid) during stress to avoid severe illness.
My friend is following this medical regimen (basically two tablets a day) and the transformation has indeed been wondrous: he is regaining weight, energy and the zest for life.