There is considerable enthusiasm for innovative battery technology for both electric vehicles and domestic solar power back-up. Central to this new technology are developments in rechargeable batteries using lithium, of which there are many types and variants. Of particular interest are developments of the lithium–air battery, which has as its basis the oxidation of lithium metal:
4Li → 4Li+ + 4e–
and
O2 + 4e– → 2O2–
4Li + O2 → 2Li2O
The interest in batteries based on this reaction is because the theoretical energy density of the battery (40.6 MJ/kg) approaches that for conventional transport fuels such as diesel (about 44 MJ/kg) and theoretically the battery can recover full efficiency following deep discharge. Efficient recharge is a problem with many other lithium-based batteries and a major issue with lead–acid batteries.
In practice, the energy density of the lithium–air battery will be less than 40.6 MJ/kg and current practice is far from this ideal; for example, the battery pack in the Tesla car (which is a complex lithium ion battery) weighs approximately 400 kg compared to a tank of conventional fuel at about 50 kg.
Simply, the lithium–air battery comprises a lithium metal anode and a graphene cathode. The cathode absorbs oxygen from the atmosphere and converts it into O2– ions, which diffuse through to a conductive layer separating the cathode and anode. Recharge is the reverse of the process. Of particular concern is that on re-charge build up of metallic bridges of lithium from the anode through the conducting layer can occur. This ultimately leads to an internal short circuit, which can cause the battery to catch fire.
If solar power back-up is to take-off as is widely predicted by proponents of this technology, there will be a major demand for lithium batteries and hence lithium metal. Australia is a significant producer of lithium ores from the Talison Lithium mine at Greenbushes in Western Australia and there are several prospects for new lithium mining ventures. These projects are in competition to extraction of lithium from brine lakes mainly in South America.
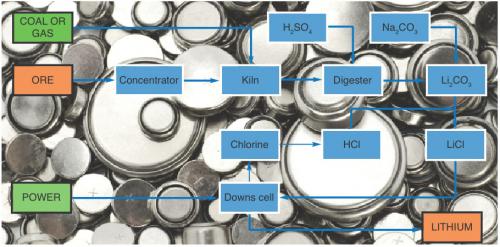
The steps in the production of lithium from ore are shown in the flowchart. The principal ore for lithium production is alpha-spodumene, which is a lithium aluminosilicate of empirical formula LiAlSi2O6; in its pure form it contains 8% lithium (as Li2O) but most productive ore contains much less, typically 1.5–4.5% Li2O. The first step is to concentrate the ore to about 90% spodumene by flotation and gravity separation. Australian processing operations generally end here and the concentrate is exported.
Alpha-spodumene is very refractory and cannot be processed to extract the lithium. The alpha-spodumene is converted into the beta form, which is less dense and from which the lithium can be extracted by sulfuric acid digestion. The transformation requires the alpha-spodumene to be heated to over 1100°C and is typically performed in a rotary kiln using coal or gas. The product is crushed in a ball-mill and after extraction with sulfuric acid, the excess acid is neutralised with limestone. Then sodium carbonate (soda ash) is added to produce a lithium carbonate solution, which is evaporated prior to the addition of more soda ash, which precipitates the carbonate. This material is widely traded and competes with lithium carbonate produced from brine lakes.
Lithium metal is produced by electrolysis of a lithium chloride/
potassium chloride eutectic at about 450°C. To perform this operation the lithium carbonate is converted into lithium chloride by the addition of hydrochloric acid. The lithium chloride is charged to the electrolysis cell (Downs cell) where by-product chlorine is also produced. The chlorine can be traded for or converted into hydrochloric acid. The molten metal is extracted and cast into ingots. This process is very power intensive, typically 35 kWh/kg. The outline economics are shown in the table.
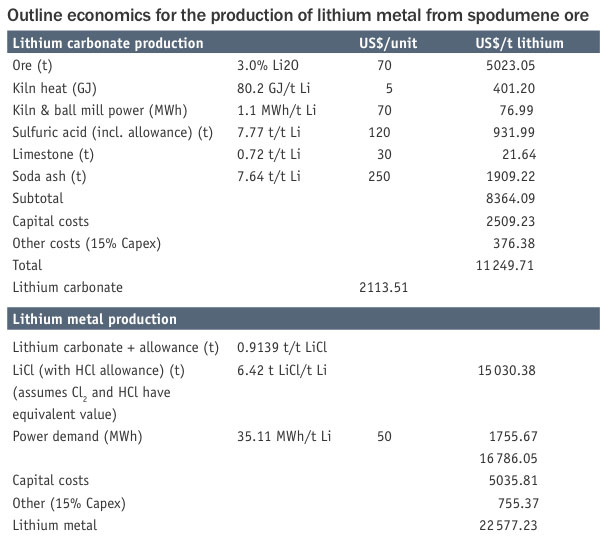
Starting with ore at a notional 3% Li2O content, the cost of production of lithium carbonate is over $2000/t, which is equivalent to a lithium value of over $11 000/t. This production cost of lithium carbonate compares favourably with recent prices for this material, which stand at over $5000/t, but the lithium carbonate may require further purification for battery grade product. Of note is the very high energy demand for this part of the process (80 GJ/t Li and 1.1 MWh/t Li). This is mainly associated with calcining the ore to produce the beta-spodumene.
Transforming the carbonate to lithium chloride and producing the metal doubles the production cost to around $22 000/t, which is also well below recent traded prices. But again further purification and metal casting may be required for the battery grade material.
Clearly producing the high valued metal is very energy intensive and increased production of lithium will be favoured by low energy prices. Since energy prices in Australia are no longer competitive with those in other parts of the world, it is highly likely that Australia’s role will remain as a provider of ore and concentrate to industrialised nations with low energy costs such as the US, China and India.
Also, if we assume theoretical efficiency of the battery, then at a notional power requirement of 20 kWh/day for an average home, the lithium required for one week’s power supply as back-up would be 12.5 kg. Allowing for efficiency losses and non-ideal operation, in practice the lithium required may be many times this amount. This is a significant amount of lithium metal to be carried by an ordinary household. Those of us who have experienced small laboratory fires involving a few grams of alkali or alkaline earth metals, may find the thought of a fire involving more than 12 kg of metal somewhat alarming. International airlines prohibit the transport of lithium batteries except under strict conditions because of their tendency to spontaneously ignite (due to internal shorting within the battery). Safety concerns about the widespread use of large lithium batteries in the household are largely ignored by the proponents of lithium battery technology and safety issues could become a major hurdle for the widespread uptake of solar/battery storage.
There are many challenging problems to be solved in the development of lithium battery technology, which will require innovative chemical solutions. Large electronics conglomerates (mainly Japanese and Korean) are becoming dominant in the development of new battery chemistry and battery manufacture and many of the vehicle manufacturers are in joint venture with them (Tesla is reported to have an agreement with Panasonic). Of relevance to Australia would be new technology to extract the lithium from alpha-spodumene at lower energy cost that the current practice.