Every time I put the recycling bin out, I think of Karl Ziegler (1898–1973) because he gave us high-density polyethylene (HDPE), one of just three polymer types that I’m allowed to put in the bin because they are the only ones the recycling company that serves our area can handle. The other two are polypropylene (PP), another tribute to Ziegler, and polyethylene terephthalate (PET). Ziegler jointly with Giulio Natta was awarded the Nobel Prize in Chemistry in 1963. In April, the Karl Ziegler Foundation, founded by his daughter and awarding prizes to young scientists, held a jubilee conference to celebrate and reflect on his life and work and to ponder the future.
On the bottom of containers are stamped the triangle identifiers. Have trouble seeing them? Try rubbing a marking pen over the stamp to enhance it. The diagram also shows the code for low-density polyethylene (LDPE), because that’s where this story begins.
The first industrial polyethylene was made in 1933 by polymerising ethylene gas at high temperatures and pressures in the presence of a free radical initiator (R•). The first step produces a new carbon-centred radical, R–CH2–CH2•, one more gives R–CH2–CH2–CH2–CH2•, and eventually we need to represent the growing macromolecular chain as R–CH2–CH2–(CH2–CH2)n–CH2–CH2• where n can be a very large number indeed. Like most simple pictures in chemistry, this one is a useful starting point but it doesn’t tell the whole story.
Free radicals don’t always do what we expect (hope?) them to do, and in this case, as the chain grows, every now and then the terminal radical doesn’t grab a passing ethylene molecule, but instead ‘bites back’ on its chain and abstracts a hydrogen atom, leaving the radical centre a few carbons back on the chain. The structure looks like this: –CH22–C•H–CH2–CH2–CH2–CH3. The chain has not finished growing, of course, but growth takes off from that carbon radical, leaving a four-carbon branch dangling off the long chain. Other modes of back-biting can occur, too, so about 2% of the carbons in the long polymer backbone carry a side chain.
In 1953, Ziegler found a new way to polymerise ethylene, using triethyl aluminium and titanium tetrachloride to produce polyethylene at low temperatures and normal pressures. The mechanism is complicated but involves the first ethylene molecule bonding to the titanium to form CH3–CH2–TiCl3, after which the next ethylene inserts into the carbon–titanium bond to form CH3–CH2–CH2–CH2–TiCl3, following which a long polyethylene chain is quickly formed.
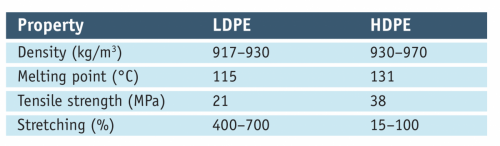
The difference in the physical properties of low-density and high-density polyethylene is small but very important (see table).
Relating chemical structure to the density of solids is a game fraught with error, but in a limited family like this (and remembering that these are not rods but zig-zag chains) it’s reasonable to expect the chains of HDPE to pack together better than their furry LDPE cousins, leading to higher density, melting point and strength. HPDE is used in many food containers, whereas LDPE is used in polymer films that also have food applications and in the screw caps of some containers. For example, both my milk and orange juice come in HDPE bottles, but the milk has an HDPE cap (over a foil seal) while the juice cap is LDPE. Go figure.
One of the clever things about the organometallic route to polyethylene is that it can be used to produce LDPE with varying degrees of ‘L’, by simply using a monomer mixture of ethylene with a higher alkene such as but-1-ene or hex-1-ene, so the resulting hydrocarbon chain has a proportion of two- or four-carbon side chains, respectively.
To return to my recycling theme, PET with its ester linkages in the chain is the easiest of the four to degrade by chemical or enzymic means, the latter being pursued by chemical engineers at ANU. Given the cost of collection and separation and the scale of operations, even successful technologies are unlikely to be economically competitive until the price of virgin material rises due to decline in the production of crude oil, or governments introduce legislation that forces manufacturers to use at least some of the recycled material no matter how much it costs. Don’t hold your breath.
There seems to be no chemical or enzymic way to reconvert the other three polymers to monomeric alkenes that can be separated from rubbish and repolymerised. The best technology on offer appears to be the pyrolysis process developed by Thomas Maschmeyer (University of Sydney). There are opponents to this and also the burning of waste plastic for energy recovery, partly on philosophical grounds (actual recycling is preferred) and partly because of the consequent production of the greenhouse bogey, CO2, but some such combustion must occur in the waste incinerators that are popular in Europe.