Dr Jennifer Doudna of the University of California, Berkeley (UCB), and Dr Emmanuelle Charpentier, formerly of the University of Vienna and now the Max Planck Institute, are the joint 2020 Nobel Prize laureates in chemistry ‘for the development of a method for genome editing’. The announcement of the Nobel Prize in Chemistry 2020 is well-deserved acknowledgement of the significant advances of Doudna and Charpentier in the field of genome editing utilising CRISPR-Cas9 technology. It’s particularly momentous when you consider that, previously, only five women had been awarded the Nobel Prize in Chemistry: Marie Curie (1911), Irène Joliot-Curie (1935), Dorothy Hodgkin (1964), Ada Yonath (2009) and Frances Arnold (2018). Also, it is the first occasion on which two women have been jointly awarded a Nobel Prize in any science category.
But there might be more to it: the awarding of the Nobel Prize in Chemistry 2020 may also be considered a political statement of sorts. For almost a decade, UCB has been locked in a patent battle with The Broad Institute (‘Broad’), home to another CRISPR forerunner, Dr Feng Zhang, over the ownership of the CRISPR-Cas9 technology. By exclusively attributing the development of a method for genome editing to Doudna and Charpentier, the highly revered Royal Swedish Academy of Sciences has, in the most public of forums, made clear who it considers to be the original developers of the technology.
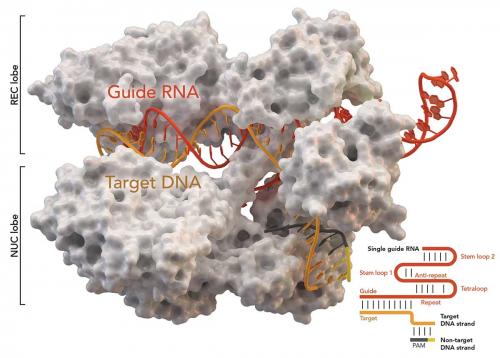
After initially meeting at a science conference in Puerto Rico in 2011, Doudna and Charpentier bonded over their shared research interest in the CRISPR systems. Charpentier had recently published a Nature paper describing the type II system (CRISPR-Cas9). It was to be the start of a collaboration that would culminate in their seminal 2012 Science paper, which demonstrated that an RNA-guided protein, Cas9, is capable of reading genetic information on CRISPR sequences and subsequently using that information to identify and destroy viral DNA. Even further, their work showed that the RNA could be engineered to target any gene, highlighting the unique ability of the CRISPR-Cas9 system to act as an elegant tool for precisely adding or deleting specific strands of DNA, effectively editing DNA in vitro.
Zhang, together with his team at Broad, published a subsequent paper in the same issue of Science. The paper described the refinement of the CRISPR-Cas9 technology, exemplifying its particular application in eukaryotes. Mere weeks later, Doudna published a paper about the ability of CRISPR-Cas9 technology to edit genes in animal cells.
It is the demonstration of this particular application in eukaryotes that forms the basis of the ongoing claims to ownership rights of the intellectual property encompassing the technology.
In 2012, UCB filed its patent directed to the use of the CRISPR-Cas9 technology for gene editing. The application of the technology in prokaryotes and in vitro was detailed, but the application potentially allowed for uses beyond prokaryotic or in vitro environments.
However, in 2013, Broad filed its patent directed to the use of the CRISPR-Cas9 technology specifically for gene editing in eukaryotes, which was supported by examples of DNA editing inside mouse and human cells. Broad successfully applied for Track One status, meaning its patent application effectively jumped ahead of UCB’s application in the examination queue. Consequently, Broad received the first issued US patent to the use of CRISPR-Cas9 technology in gene editing in eukaryotic cells in April 2014. UCB’s patent application remained in the examination queue. In essence, despite UCB being the first to file its patent applications, the Broad patent was preferentially issued.
Broad’s issued patent represented a highly valuable commodity in the commercialisation of the CRISPR-Cas9 technology in a range of applications in biotechnological, pharmaceutical and agricultural fields.
At the time both applications were filed, US patent law was founded on the ‘first to invent’ principle. This meant that, in the instance of two or more patent applications claiming the same or substantially similar subject matter, regardless of when a patent application was filed, entitlement to the invention flowed to the first to have invented the claimed technology.
A party could challenge an Applicant’s entitlement to a claimed invention by filing interference proceedings. The purpose of the proceedings would be to establish which party first invented the claimed invention. Accordingly, to challenge the grant of Broad’s patent, UC launched interference proceedings before the US Patent Trial and Appeal Board (PTAB), asserting that Doudna and Charpentier were the first to invent the CRISPR-Cas9 technology. Essentially, they argued that Broad had ‘interfered’ with UCB’s patents in that Broad was not the first to invent the claimed invention.
In its arguments, UCB claimed that Doudna’s work acted as a platform for Zhang’s work – Doudna had indeed anticipated that the CRISPR-Cas9 technology would find application in eukaryotic cells, and was therefore the first to invent the technology.
In rebuttal, Broad’s argument was three-fold.
First, the inventions cannot be considered the same because UCB’s is directed to the use of the technology in prokaryotes/in vitro, and Broad’s is directed to the use of the technology in eukaryotes. Further, the UCB patent application did not teach how the technology could be adapted from a prokaryote/in vitro environment to a eukaryote environment, and Broad argued that it needed to overcome a significant amount of difficulties in order to apply the technology in eukaryotes. Ultimately, it is therefore a different invention from that of UCB’s patent application, and therefore Broad cannot be considered to have interfered with UCB’s patents.
Second, even if the UCB patent application is considered to extend to eukaryotic applications, Zhang was the first to invent the application of the technology in eukaryotes, having worked on the technology since February 2011.
Third, Broad claimed that Cas9 itself is a naturally occurring protein, and therefore does not meet the criteria of patentable subject matter. In contrast, Zhang’s application in genome editing of eukaryotic cells is more than mere discovery, and constitutes patentable subject matter.
Initially, no firm decision as to interference was made by the judging panel. UCB and Broad were therefore presented with three options. On the one hand, if Doudna was found to be the first inventor, UCB would effectively be entitled to Broad’s patent. On the other hand, if Zhang was found to be the first inventor, Broad would maintain its issued US patent. As an alternative, Doudna and Zhang could be recognised as co-inventors.
Eventually, the PTAB decided in 2018 that there had been no interference-in-fact. The decision was centred on the finding that UCB’s patent application and Broad’s issued patent were directed to two, separate inventions. The reasoning for this is that while UCB’s application may have suggested that CRISPR-Cas9 could be used in environments other than prokaryotes/in vitro, such as a eukaryote environment, the PTAB decided that it would not have been obvious to do so. That is, Broad’s patent application is inventive in its own right.
UCB appealed the decision and, after the latest round, the PTAB found in September 2020 that Broad maintains ownership of its patent. However, the saga remains ongoing, with the PTAB requesting that UCB present additional evidence that it had demonstrated CRISPR-Cas9’s application in eukaryotic cells prior to Broad.
The patent battle is not isolated to the US. Proceedings are also underway in most major jurisdictions, including before the European Patent Office and the Australian Patent Office.
The dispute has turned nasty, extending beyond the US Patent and Trade Marks Office. President and Founding Director of The Broad Institute, Eric Lander, penned a 2015 Cell article titled ‘The heroes of CRISPR’. Lander subjectively claimed that Broad scientists developed the CRISPR-Cas9 technology, a statement that diminished the efforts of Doudna and Charpentier. Three scientists consequently came forward to dispute several key facts in the article, forcing Lander to later add ‘clarifications’. In response, biologist Michael Eisen of UCB claimed the article was ‘science propaganda at its most repellent’, and was among many to call for its retraction. At an extreme, it was claimed the article was a man’s attempt to ‘write women out of CRISPR’ (bit.ly/3579Ysp). The Twitter hashtag #landergate was born.
The ongoing dispute highlights the potential commercial value of intellectual property. Indeed, many companies have since been founded on the CRISPR technology. Exclusive licenses of the UCB patents have been granted to at least Caribou Biosciences Inc., Intellia Therapeutics, ERS Genomics, Casebia Therapeutics and CRISPR Therapeutics AG. Similarly, Editas Medicine is associated with Broad’s patented technology.
Ironically, during this dispute of almost decade in relation to ownership, the commercial value of those originating patents is diminishing. With neither party able to fully capitalise on the originating technology, ongoing scientific work has since established a series of other, commercially relevant, CRISPR systems, including CasX, CasY, Cas12 and so on.
The ongoing intellectual property ownership dispute of the CRISPR-Cas9 technology has undoubtedly detracted from the science at its core – a novel, revolutionary technique for gene editing.
The awarding of the Nobel Prize in Chemistry to Doudna and Charpentier is, undeniably, a refreshing reminder of scientists doing science for science’s sake. Doudna and Charpentier, together with their team, have irrefutably made significant contributions to CRISPR-Cas9 technology and the scientific community’s understanding of genomic editing. Their research has spurred, and will continue to spur, further innovations in the field.