Globally, there are more than enough sustainable energy sources to supply the world’s electricity demands. The issue is that sun, wind and hydropower resources are often found in rural locations and the energy is not easily ‘dispatchable’ as needed to cities, where demand is localised. Batteries form part of the solution but are expensive, require scarce metals for manufacture and are too heavy to represent a transportable source of large amounts of energy.
A recently emerging concept aims to store energy derived from these natural resources in the bonds of ammonia molecules. Ammonia is ideal for transport by ship or pipeline as liquefied energy (liquefies at 10 bar at room temperature or at –33ºC at 1 bar; lower heating energy 5.2 MWh/tonne). Conveniently, the practice of liquid ammonia transportation is already commonplace in the fertiliser industry – 175 million tonnes were produced worldwide for a market value of US$70 billion in 2019, according to the United States Geological Survey (on.doi.gov/3grUUbY). So, mature technologies and infrastructure already exist to help allow this to develop.
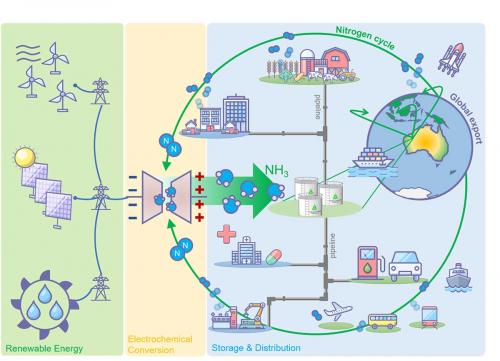
Given Australia’s massive potential in renewable energy resources, ammonia is rapidly emerging as an ideal export vector that can be readily shipped to market. As we will explain here, ammonia can thereby feed the hydrogen economies that are springing up in several countries, but has great, even surprising, potential as a fuel in its own right. In our recently published ammonia roadmap article (MacFarlane et al., doi.org/10.1016/j.joule.2020.04.004), we detail pathways towards scale-up, research progress and a multitude of direct end-use modalities, and discuss the parallel need for further understanding of the potential impacts on the global nitrogen cycle.
Most of today’s ammonia is produced by the Haber–Bosch process. Developed more than 100 years ago, it generates a very large carbon footprint, relies on fossil fuels such as methane and is efficient only at large scale in facilities that run at full capacity. We identify in our roadmap three distinct generations of technology that will move the industry away from Haber–Bosch, towards the sustainable generation of ammonia, using water, air and energy from intermittent renewable energy sources. These processes have the potential to be efficient on all scales, from a small family farm, to enormous solar- and wind-powered facilities in the Australian outback.
Although the Haber–Bosch process has been optimised over time, high temperatures and pressures (>400°C and >200 bar) are still required for efficient ammonia production. That being said, the main culprit for the large carbon footprint is one of the starting materials: high-purity hydrogen gas, usually generated by steam-reformed methane. Currently, the Haber–Bosch process produces ammonia at an energy cost of 8 MWh/tonne and 65% energy efficiency, accounting for 1–2% of global energy consumption and more than 1% of global greenhouse gases.
Generational steps towards renewable ammonia
To improve this environmental impact, Generation 1 Haber–Bosch plants can operate with carbon capture and sequestration or the purchase of carbon credits. This represents an expensive but transitional step for the industry and infrastructure. The product is known as blue ammonia.
Generation 2 renewable ammonia refers to the product of Haber–Bosch ammonia plants being fed hydrogen that is generated by water-splitting electrolysers, instead of steam methane reformation. Producing ‘green ammonia’, this technology, like Generation 1, avoids mothballing current Haber–Bosch infrastructure. In the UK, Siemens’ pilot plant demonstration produces 30 kilograms of ammonia per day, powered by wind energy fed to a water electrolyser (sie.ag/39XlY07). The capital cost of a proton exchange membrane electrolyser for a conventional Haber–Bosch plant is US$1 million per megawatt but this cost is expected to decrease with further R&D. Another option is high-temperature solid oxide electrolysis, which uses waste heat from the Haber–Bosch process to split water as discussed by Danish catalysis company Haldor Topsoe (bit.ly/3fr4uKV). High-temperature solid oxide electrolysis, like a Haber–Bosch plant, is challenged by the intermittent nature of renewable energy while proton exchange membrane electrolysis is not to the same extent. Nonetheless, some form of hydrogen storage is an important requirement to smooth the intermittency of H2 supply into the Haber–Bosch process.
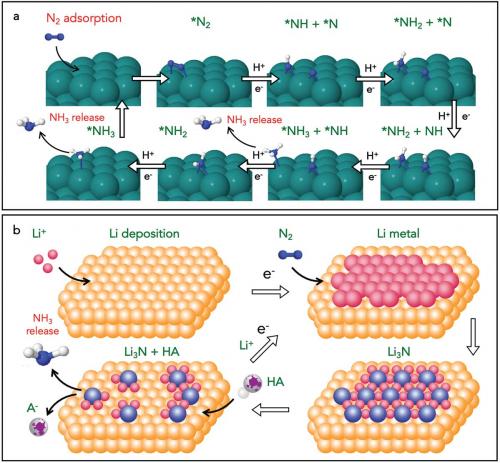
In Generation 3, the Haber–Bosch plant is no longer required and the benefits include resilience to intermittency of power supply, versatility of scale and lower N2 purity requirements. Ammonia is produced by the electroreduction of nitrogen at a selective cathode, along with a proton source (e.g. water by oxidation at the anode) and intermittent renewable energy as the power supply. Research is currently directed to two main pathways:
- the direct electrochemical nitrogen reduction reaction (eNRR)
- an indirect eNRR mechanism using a redox mediator.
This latter, appealing method can potentially produce ammonia at an energy cost of 15.5 MWh/tonne (NH3) and research effort is focused on lowering this further.
Targets and challenges of Generation 3
Reduction of the triple bond in the N2 molecule is very difficult (hence the inert nature of N2 gas). Researchers can easily mistake the reduction of oxidised nitrogen species such as nitrate, nitrite, N2O and NO (collectively termed NOx) that are ubiquitous in the atmosphere, on laboratory consumables, or from commercially supplied gas cylinders and chemical reagents for eNRR. Meticulous cleaning, purification and scrubbing procedures are required to ensure that only N2 is present. Isotopically labelled experiments with 15N2 and NMR studies are also crucial. To this end, we (doi.org/10.1038/s41929-019-0252-4) and other groups have published protocols for conducting eNRR experiments. Unfortunately, recent peer-reviewed articles often do not include robust quality controls, leaving the literature littered with question marks and false positive results. This is particularly true of studies of aqueous eNRR systems because the yields are often low and competition from the proton reduction to hydrogen reaction is severe.
Higher ammonia yields (approaching 100 nmol/s/cm2) and Faradaic efficiency (>88%) are commonly achieved by the indirect redox-mediated eNRR method, placing this method in a more secure regime in terms of robust results.
Practical challenges of scaling up Generation 3
There are a number of practical challenges to scaling up Generation 3 approaches. Purified nitrogen starting material produced by air separation is a valuable commodity and thus separation of the produced ammonia followed by recirculation of the nitrogen is necessary but adds capital cost.
Stoichiometrically, 1.5 tonnes of water is required per tonne of ammonia produced. Often, in areas where solar power is abundant, water is not, so this is an important consideration for large-scale eNRR. Sea water could be used directly for the supporting anode reaction either via the chlorine evolution reaction (chloride to chlorine) or remain as the water oxidation reaction (water to oxygen), which is used for Generation 2 technologies. The issue with the former is that chlorine will be produced at 20 times global demand, with obvious environmental implications. The issue with the latter is unearthing an electrocatalyst that will perform the water oxidation reaction with the selectivity and durability required under saline conditions.
Although both Generation 2 and 3 technology can operate intermittently according to the availability of renewable energy, decreasing the capacity factor will lead to an increase in capital cost per tonne of product. Storage of energy, whether in a battery (present cost US$300 000/MWh stored) or as compressed hydrogen, will also increase the capital and production costs significantly.
From a social acceptance perspective, most people would immediately associate ammonia with its awful smell, which links to a perception of high toxicity. The smell can actually be viewed as a blessing in disguise: the human nose can detect ammonia at 5 ppm, which is five times lower than the concentration determined to be harmful (threshold limit value 25 ppm). Odourless gases such as hydrogen and carbon monoxide can leak undetected, resulting in increased flammability or suffocation risks. Ammonia is not classed as carcinogenic, unlike petrol. Early studies and theses advised that the risks of using ammonia as a fuel were comparable to or lower than those of liquid petroleum gas. In the maritime sector, a study determined that once mitigation strategies were in place, the risk of catastrophic failure from the use of ammonia fuel became extremely low (bit.ly/3gqE25t). Anhydrous ammonia is already transported in large amounts around the globe for the agricultural sector, and no fundamental barriers are apparent with increasing this by orders of magnitude. It is, of course, crucially important that continued research and development of procedures and standards for handling and transport take place. The Ammonia Safety Training Institute is an important leader in this sector.
End-use modalities
Excitingly, CSIRO has already developed the catalysts to crack ammonia into nitrogen and hydrogen gas for use in the hydrogen economy. In this sense, the ammonia and hydrogen economies overlap in synergistic ways.
On the other hand, ammonia has the potential to be used directly as a fuel source (see image page 18) and this idea has stimulated intense research and development. Surprisingly, viewed on a cost per unit of useful energy in a variety of transportation applications, ammonia already competes quite well.
End-use modalities already demonstrated include vehicle and marine engines, particularly in hybrid fuel internal combustion engines. Ammonia for use in power generation is being demonstrated at small scale (e.g. as a replacement for diesel generation) and large scale (e.g. supplementary fuel in gas- and coal-fired power plants in Japan). Interest is high in using ammonia directly in gas turbines for power generation. This development has also led to ammonia being considered as a jet fuel. A study on this topic concluded that ammonia would be similar to traditional Jet A-1 in terms of performance, while decreasing NOx emissions (doi.org/10.3390/en11020392).
Lastly, ammonia can be used directly in solid oxide fuel cells and direct ammonia fuel cells to produce electricity. The former operates at high temperatures to ensure conversion efficiency and is forecast to be used in applications with high energy demand (e.g. the marine sector).
Although catalytic converters already used with traditional fuels are commonly transferrable to this sector, it is important to note that NOx emissions from ammonia combustion do need to be carefully monitored and treated. This topic is already clearly in focus in most development studies.
Increased ammonia use and the Earth’s nitrogen cycle
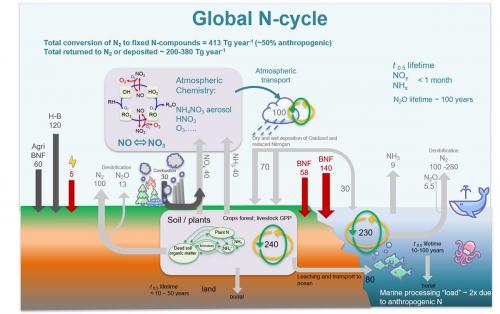
To correct the present imbalance in the Earth’s carbon cycle generated by excess CO2 emissions, only to create a similarly damaging imbalance in the nitrogen cycle, would be counterproductive. Through ammonia production for the agricultural sector via the Haber–Bosch process, anthropogenic nitrogen fixation already accounts for double that of natural processes. Although planned as a circular economy where NH3 releases energy when cracked back into N2 and H2O, extreme caution is required, as even small adventitious losses of reactive nitrogen/NOx side products will accumulate substantially due to the scale increase. Compared with the planetary carbon cycle, less is understood about the nitrogen equivalent. Nitrogen is fixed at a rate of 413 Tg/year of which 50% is from anthropogenic sources; 200–380 T/year of the fixed nitrogen is accounted for and returns to the unreactive form N2, leaving 33–213 T/year unaccounted for. Knowledge gaps about the load processed in the deep ocean, where N2O, a powerful greenhouse gas, accumulates with a half-life of 100 years, require thorough scientific study. Further, limited information is available regarding denitrification processes in the atmosphere.
Ammonia has ideal chemical properties to become a significant, easily transportable fuel for the supply of energy globally. Producing ammonia without carbon emissions is possible and the roadmap that this article summarises describes the pathway towards this in terms of three generations, the third of which is the electrochemical approach. Although faced with challenges, this latter method breaks the reliance on traditional, inflexible Haber–Bosch technologies. Ammonia can be viewed as a hydrogen carrier; however, many direct-use modalities are also at various stages of development and conventional fuels can be replaced by ammonia with similar or improved safety. The future of ammonia as an ideal store of renewable energy and fossil fuel replacement is rapidly becoming an exciting reality and as a result has the potential to become a massive renewable energy export industry for Australia.