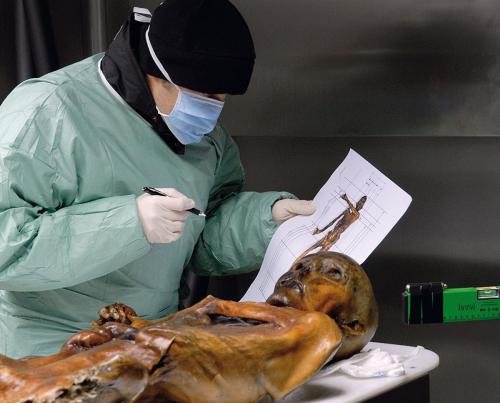
While hiking in the southern Austrian mountains very close to northern Italy in September 1991, Erika and Helmut Simon stumbled across the top half of a human corpse protruding from glacial ice. Once notified, local authorities thought it was a hiker missing in the area from some years back and they ‘hacked’ the corpse out of the ice, initially with a jackhammer, resulting in it sustaining some damage. Along with some then-unidentified materials that were collected and bagged, the corpse was taken for closer inspection to the University of Innsbruck.It became quickly evident that the corpse was not a recent victim of the mountains but rather, it was many thousands of years old, having been dehydrated, preserved and mummified by the ice that encapsulated it. It was another case where post-mortem decomposition processes were slowed by the environment surrounding the corpse (see May 2018 issue, p. 18). The materials collected at the site were found to be personal artefacts of the mummy, comprising clothing, shoes, hunting equipment and a variety of other implements that a mountain-dwelling Neolithic Homo sapiens would evidently have.
It became apparent that the mummy was one of the most significant archaeological finds of the century. As this information came to light, the world’s attention heightened. What was to become of this man in the ice? His future firstly depended on one fundamental question – was he found in Austria or in Italy? This was important as the mummy was clearly of great scientific and monetary value.
The mummy, dubbed ‘The Iceman’ by the press, was found on the very edge of the Ötzal Valley of Austria, and christened with the descriptor of an inhabitant of that valley – Ötzi (pronounced Ertzi). The border in this region has been disputed for centuries, with the current agreement signed in 1919 after World War I depending on the line between the highest rock ridges in the area – literally the watershed where rainwater flow is split between the two adjacent valleys.
While the initial discovery was thought to be on the Austria side of this border, subsequent excavation of the site revealed that the glacial ice in the area had retreated to unprecedented levels that summer, and the underlying rock ridges had taken a slightly different course than expected. This meant that the mummy was actually inside the Italian border by 93 metres, in the Schnalstal Valley. The glacial mummy should therefore have been renamed Schnalzi, but it was too late as the original name had stuck.
The survey also revealed that Ötzi’s preservation was a stroke of luck. He had been pinned under a rocky ledge and was protected from the inexorable glacial flow, which would have otherwise crushed him.
The border dispute settled, Ötzi was transported from Innsbruck University in an ambulance under police and helicopter escort across the alpine watershed to the new, purpose-built South Tyrol Museum of Archaeology in Bolzano, Italy (see www.iceman.it). I have been reliably informed that Austrian scientists who had looked after Ötzi shed tears during this episode, while others were not happy about the deliberations that had resulted in this terrible loss.
A high-tech refrigerated showcase was constructed for the new Italian, but the first problem encountered was that Ötzi was dehydrating and losing weight at an alarming rate. Museum scientists were very concerned about their prized specimen, even more so the Museum’s management after spending so much money on their new facility. Refrigerated storage of mummies had to be re-invented so that Ötzi would be preserved for posterity at a glacial –6°C and 98% humidity to prevent his desiccation. The observation facility is in a quiet corner of the Museum where visitors can pay due respect to the mummified remains.
The life and death of Ötzi
So why is Ötzi, a 5300-year-old mummified Neolithic European found in a rocky gully 3200 metres in the Alps, so special? Apart from the altitude at which he was found, which was believed to be impossible for Neolithic humans to travail, the technology of his extensive clothing and implements has caused a virtual bonfire of the textbooks to that time.
The Neolithic was a period of tremendous human development, commencing with the beginning of farming about 10 000 BCE and ending with the smelting of ores to obtain metal for tools between 4500 and 2000 BCE. Ötzi was a Copper Age Neolith, possessing both tools made from stone and the first metal humans learned to smelt – copper.
He was not a young man for a Neolith, being about 46, but he must have been fit, strong and very experienced in the skills needed to travel alone in mountainous terrain at altitude. For example, he carried birch bark containers in which he stored glowing charcoal embers that enabled him to start a fire quickly to cook and keep warm. He was very well clothed, having different layers, including leggings, a hefty woven grass cape and intricately woven shoes stuffed with straw for insulation.
He was also very well armed, carrying a small, sharp flint-bladed dagger, a hefty axe with a beautifully finished copper blade, and a longbow with a quiver of arrows.
A large variety of Ötzi’s personal possessions were also found with him, comprising an incredible array of implements and materials to ensure his survival. These included medicinal plants, highly nutritious berries, an antler sewing needle and string among many other items of equipment that he packed into a belt pouch and a rucksack.
Closer inspection of Ötzi’s body found that it was covered with about 60 tattoos made by rubbing charcoal into tiny skin cuts.
However, all his technology and endurance did not stop someone shooting an arrow into his back – sensationally found by X-ray imaging 10 years after the original discovery – resulting in his death at his alpine resting place.
The isotope chemists
Ötzi’s glacially mummified body has been the subject of intense scientific investigation using a vast array of instrumentation and techniques, including the analysis of a number of isotopes. For the non-scientist, isotopes are forms of an element with slight differences in atomic structure in the central nucleus; they are the same element and occupy the same position in the periodic table, but all have slightly different chemical and physical properties.
A prime example of the usefulness of this quirk of nature is 14C radiogenic dating, which was used to determine Ötzi’s time of death. The technique was developed by Willard Libby in the late 1940s at the University of Chicago, for which he received the 1960 Nobel Prize in Chemistry. It is based upon the continuous production of a radioactive isotope of carbon (14C) in the atmosphere by the interaction of cosmic rays with atmospheric nitrogen. Subsequent reaction with atmospheric oxygen results in the formation of radioactive carbon dioxide. This becomes incorporated into plants via photosynthesis and into the animals that consume them. These processes result in an equilibrium mixture in living plants and animals of 14C with other stable isotopes of carbon (mainly consisting of 12C), and is essentially constant over time. When an animal or plant dies, it stops exchanging carbon in its environment and the amount of 14C in the organism decreases by radioactive decay.
With a half-life of 5700 years, 14C techniques can date preserved animal and plant samples to ten half-lives, or approximately 60 000 years. Because this period covers a tremendous change in human development, it has been referred to as a ‘gift of nature’ to archaeology.
The downside is that 14C’s low concentration makes its analysis a challenge – its relative amount in the carbon pool is in the order of one part per thousand billion (1012). Historically this has required high sample weights, but modern mass spectrometry techniques using ion acceleration have reduced sample requirements to milligrams.
The samples used to date Ötzi came from his damaged left hip (following the jackhammer episode) as well as grass from his straw cape and left shoe. The finding that about half of the 14C in his body had radioactively decayed revealed that Ötzi died and was cocooned in glacial ice 5300 years ago. This would place Ötzi’s age at around 3300 BCE, well before the construction of the Great Pyramid at Giza (about 2570 BCE) and at about the time when Stonehenge construction began (about 3000 BCE).
Another treasure trove for isotope chemists is the teeth and bones of any subject, which encapsulate an isotopic trail about where they grew up, travelled, and where they are now. Ötzi’s teeth have been extensively analysed, in particular the enamel, which is an outer layer of mainly calcium phosphate that formed in early childhood. As calcium is deposited, so too is its ‘group 2 chemical cousin’ strontium, which comes from rocks, and is a common constituent in soil via weathering and thus the food grown from it, as well as water that has been in contact with the rocks.
Once it is incorporated in tooth enamel during early childhood, the strontium becomes sealed and does not exchange with any further ingestion of the element. This makes things interesting, since strontium exists as a mixture of isotopes that are particular to the rock from which it was derived. Therefore, when the young Ötzi was thirsty, he would have drunk from mountain streams and springs and incorporated strontium into his system. As his new teeth set, the isotopic fingerprint of the strontium he had ingested was preserved.
The archaeological isotopic ratio of interest is 87Sr/86Sr, the latter being stable while 87Sr is formed from rubidium-87 by radioactive decay. This ratio varies significantly with rock age and chemical composition, and for the most part is different for different rock bodies. Analysis showed that the ratio of strontium isotopes in Ötzi’s tooth enamel most closely matched rocks south of the alpine watershed. (Archaeologists in the future will hopefully be aware that many of us drink bottled water from exotic places and won’t place our childhoods in the French Alps!)
To further determine more precisely where Ötzi spent his early childhood, another favoured suite of the isotope chemist was also analysed. Lead exists as four stable isotopes with mass numbers 204, 206, 207 and 208. The twist in this quartet is that all but the first come from the radioactive decay of uranium and thorium, both of which have different half-lives.
Lead is a common constituent of rocks, and their different ages and compositions provide a characteristic lead isotope fingerprint for each rock type. Once again, ingestion of food and water in contact with these rocks incorporates a lead isotope fingerprint in the tooth enamel during early childhood.
The diverse rocks and water streams around Ötzi’s discovery site have been extensively sampled, and their strontium and lead isotopic composition determined and compared with Ötzi’s tooth enamel. The isotopic fingerprints of the tooth enamel match rocks in the vicinity of the discovery site, most closely in the Eisack Valley in Italy approximately 60 km south-east of the discovery site. Some archaeological relics from Ötzi’s time have also been found there.
Isotope fractionation
The bane of isotope chemists is that when isotopic mixtures are stored, chemically treated, transferred from one container to another or manipulated in some way, one isotope may be enriched or depleted (or so-called fractionated). However, this has been used as a positive by archaeological isotope chemists, especially in the case of heavy-oxygen water. Oxygen exists as a number of isotopes, three of which are stable: 16O (99.76%), 17O (0.04%) and 18O (0.20%). This results in a significant amount of ‘heavy’ water (H218O) with slightly different physical properties from those of normal water (mainly H216O).
In a rain event, the heavier isotope (being less volatile) is enriched in the rainwater while the remaining water vapour is depleted of this isotope. This fractionation is more pronounced as the distance from the source of the rainwater and the actual rainwater increases, as successive rain events during the water vapour’s movement results in successive depletions of the heavier H218O.
Fractionation is amplified at altitude, such as where Ötzi was found. This is especially the case in the area around the alpine watershed between the Ötzal and Schnalstal valleys, as areas north of the watershed mainly capture precipitation from the more distant Atlantic Ocean, while areas south of the watershed capture precipitation from the closer Mediterranean Ocean. The resulting rainwater in areas south of the watershed has an 18O/16O ratio slightly higher than in areas north, since it has experienced less rainwater fractionation events.
Just like lead and strontium isotopes, the oxygen from the water consumed during early childhood is also incorporated and sealed in the dental enamel. Analysis of Ötzi’s tooth enamel showed that as a child he drank water from south of the alpine watershed, in line with the findings from the strontium and lead isotopes.
However, analysis of Ötzi’s bone samples, which are remineralised with ingested substances every 10–20 years, showed oxygen isotope values that were closer to the more northerly discovery site. This indicates that he may have spent time in a more northerly location after childhood.
Getting down to the nitty gritty
I hope that Ötzi wasn’t a private person because, apart from knowing where he grew up and travelled, the whole world now knows about his final meal. His stomach contents have been analysed by a variety of techniques, such as DNA fingerprinting, which show that his hearty last meal comprised mainly ibex, red deer and einkorn wheat.
I have hiked the mountains near the discovery site and have seen ibex perched precariously on high rock ledges. They are impressive, big, hairy, goat-like animals with huge horns; I’m not sure how I’d go hunting one of them with a bow and arrow!
Ötzi’s intestinal contents have also been examined in detail and revealed 12 small (100–400 µm) fragments of mica, a rock used commonly to make cereal grinding equipment. Ötzi would have incorporated these mica fragments while enjoying his cereal meals and/or from gritty drinks from the local water sources.
A common constituent of mica is potassium (K), which exists as a number of isotopes in nature. Two of these are stable: 39K (93.3%) and 41K (6.7%). One is radioactive, 40K (0.012%) having a half-life of 1.25 billion years. One of the radioactive decay products of 40K is argon-40 (40Ar), which is the most common isotope of argon (99.6%). Because of the long half-life of this K–Ar system, it is characteristic of many rocks and can be used to date them.
Mass spectrometric techniques have evolved to such an extent that this analysis could be performed on the tiny mica fragments found in Ötzi’s intestine. This showed that the micas were from the Italian Vinschgau area approximately 20 km south of the discovery area.
The isotopic data indicates that Ötzi grew up south of the Austrian–Italian watershed in the Eisack Valley, and as an adult spent time in mountains of the Vinschgau before setting off for his final journey in the Ötzal Alps (doi.org/10.1126/science.302.5646.759b and doi.org/10.1126/science.1089837).
The copper for Ötzi’s axe
Hatchets, axes and the like have been found in many archaeological sites, but Ötzi’s is still one of the most spectacular of the artefacts found with him. It is in ‘as new’ condition, complete with wooden handle, rope attachments and pitch tar to hold the 99.7% copper blade in place. It is the oldest implement of this type ever found in its complete form.
Non-destructive analyses showed that the axe was cast from a bivalve mould. The metal was not hardened, even though the technology was available at the time, presumably because Ötzi favoured malleability over hardness.
The provenance of the copper was a question that could not be answered by non-destructive techniques. Historical copper and copper ores from all over Europe have been extensively fingerprinted in terms of composition and trace elements present, so all that was required was an actual sample of the precious axe’s copper. After extensive consultation, this was allowed in 2016, and three microsamples of total weight 6.7 mg were removed from the blade and analysed to determine the lead isotope signature and trace element profile.
The composition of the axe blade was similar to a number of copper ores known to be used in Ötzi’s time, but what clinched its origin was that the axe’s copper contained low amounts of antimony (doi.org/10.1371/pone.0179263).
All the information pointed to copper ore deposits in southern Tuscany. This example of the drive to travel and trade so early in human technological development was a surprise to many anthropologists.