Thermal conductivity
What do you notice when you open your dishwasher after it has just finished and is still hot? The plastic containers are cool to touch but still have water droplets sitting in them. Meanwhile, the ceramics and glass plates are moderately hot and dry while the stainless steel pots and pans and cutlery are very hot.
Before you blame the dishwasher, consider this. The differences are mainly due to the thermal conductivity of the materials – the ability to transfer heat to water or to your fingers. The low thermal conductivity of plastics means they do not supply enough heat to evaporate the water on them effectively. As well, water sits up as droplets on plastic, so they have less surface area, but it spreads out on clean glass and metal.
Hot pots
Glass and ceramics are intermediate heat conductors and metals are generally good heat conductors. To test the theory, pour some boiling water (adults only) into a standard (non-induction) stainless steel pot or stovetop espresso bottom unit. The sides aren’t too hot because stainless steel is a relatively poor conductor of heat. However, the base is very hot!
The bases of these pots have a veneer of stainless steel coating on both sides of a thicker layer of either copper or aluminium, both of which are excellent heat conductors. Pots for induction stoves have an extra base that allows an induction current to heat it directly, and this material is also attracted to a magnet.
Cool jewels
A first pass for testing minerals and gemstones is to touch them to assess their thermal conductivity. You could ask to touch the many-carat stone in the special cabinet at the jeweller’s (purely for the purposes of science), to check whether it is cool. The staff reaction is probably going to be likewise.
Surprisingly, diamond holds the record for thermal conductivity, with aluminium oxide (ruby and sapphire) quite high in the rankings.
Thermal and electrical conductivity generally go hand in hand for metals, but not for non-metals. Thus diamonds, rubies and sapphires are very good electrical insulators. Heat is transferred in solids by vibrating atoms, namely via resonant sound waves called phonons. In contrast, it is weakly bonded electrons that carry electricity.
This is all very sound science and is terribly important when designing ever-smaller electronic components, to ensure that they don’t overheat.
Magnetic personalities
There is a huge variety of stainless steels. The common or kitchen variety is 18:8 Cr:Ni, and for our purposes we’ll assume its thermal behaviour is roughly similar to that of standard iron carbon steel.
Test some cutlery with a (fridge) magnet. For cheap cutlery, the knife and handle are both attracted to the magnet. For standard cutlery, the blade is attracted but not the handle. If neither the blade nor the handle is attracted, you probably have a butter knife. Sometimes some residual permanent magnetism is induced. Other stainless steel objects that need to be sharp, such as scissors, will also be magnetically attractive.
The composition is generally the same for the steel parts but the heat treatment has been different, although in some cases you might see a thin line where two pieces may have been joined.
Oranges and grapes
Atoms, like oranges, pack closely together in a number of different ways. Iron with additives provides several examples, depending on heat treatment and composition.
Ferrite and austenite adopt two of these equally efficient atomic packing arrangements (see February issue, p. 38). Below 910°C, iron has a body-centred cubic (bcc) ferritic packing structure, whereas above that temperature (but below 1400°C) it has a face-centred cubic (fcc) austenite structure.
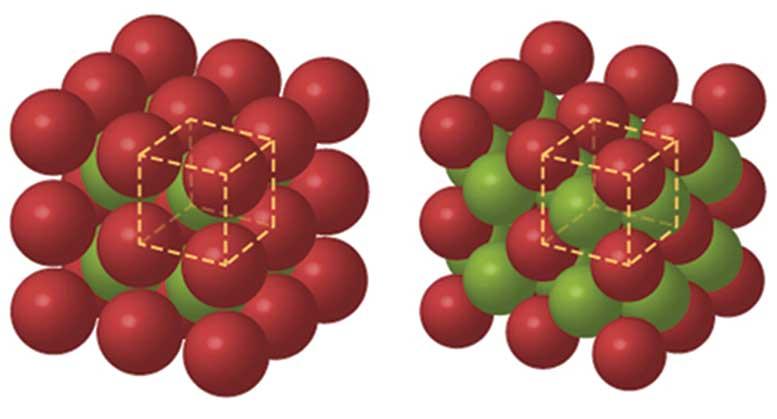
LibreTexts/CC BY-NC-SA 3.0 US
Body-centred cubic structure (left) Face-centred cubic structure (right)
In the holes between the large iron atoms, you can fit small atoms of carbon, like placing grapes between packed oranges. This carbon can also combine with iron to form iron carbide, cementite (Fe3C). More carbon can dissolve in the high-temperature austenite form than in the low-temperature ferrite.
If austenite is cooled slowly, it forms a banded structure of pure iron (ferrite) and cementite that is tough and strong but not particularly hard. Examples are knife handles, non-inductive pots and pans, kettles and the kitchen sink. However, if it is cooled quickly, the carbon atoms do not have sufficient time to move to form cementite and become trapped. This quenching is called martensite hardening and is used for knife blades, scissors and fridge cladding.
Why the first form is non-magnetic and the second is magnetic is a wee bit more complicated. Have fun roaming with a magnet.


