Any surface immersed in the sea becomes the potential home for myriad organisms, from bacteria and microalgae to corals and giant kelps. Ship and boat hulls are no exception – within minutes of immersion, the biofouling process begins.First is a conditioning phase in which organic and inorganic matter is adsorbed onto the surface. Then, within hours, small bacteria begin primary biological colonisation, followed by secondary colonisation by stalked or filamentous bacteria, diatoms and other microalgae, and protozoans. Multicellular algae and invertebrates begin to settle within days and, in the absence of any antifouling agent, the growth will cover the surface. It then builds in complexity, with slow-growing, long-lived organisms displacing the initial fast-growing, short-lived opportunists, organisms growing on organisms, and mobile organisms occupying the interstices within the growth mass.
Although essentially the same process takes place on natural substrates, the term biofouling largely refers to where the growth is unwanted, such as on immersed maritime structures and vessel hulls. On ships and boats, biofouling growth is not only unsightly but, more significantly, it impacts on vessel performance by increasing surface drag with a consequent increase in fuel consumption. Biofouling increases financial costs associated with this fuel use, and environmental costs also rise as a result of increases in gaseous emissions, including nitrous and sulfur oxides, carbon dioxide and other greenhouse gases, and particulate matter. Carbon dioxide emissions from shipping have been calculated to amount to more than 2.5% of global CO2 release. Biofouling can also accelerate corrosion of metallic substrates and translocate invasive marine species.
Attempts to prevent biofouling on vessel hulls date back to at least the Phoenicians who, at around 400 BCE, applied a mixture of arsenic, sulfur and oil to the sides of the vessels. The Greeks applied tar and wax to ships’ bottoms in the third century and, by the 13th–15th centuries, pitch, oil, resin and tallow were in use. However, despite these efforts, vessels still needed to be beached every few months to enable manual removal of fouling growth.
The first authenticated protective coating applied to a vessel was copper sheet attached to the wooden hull of the naval frigate HMS Alarm in 1758. The success of this led to the general use of copper sheathing until, with the introduction of iron ships in the early 19th century, a new solution was needed because of the corrosive effect of copper on iron.
The solution was antifouling paints, and the first practical compositions of hot soap containing copper came into widespread use in the latter 19th century. Over the next century, copper-based coatings became the mainstay of antifouling protection, but effective life rarely exceeded 12 months. The search for longer lasting and more effective coatings therefore continued. Among the advantages of antifouling coatings were ease of manufacture, ease and low cost of application, durability and applicability to many structural forms and substrates. Disadvantages were the limited life, the necessity to regularly dry-dock vessels for cleaning and repainting, the need to remove old coatings prior to new coating applications, and the tolerance of some biofouling organisms to copper.
The effect of antifouling coatings is through the continuous release of active biocide through the paint surface to kill or deter settling organisms, and this release rate must be maintained above the critical level to inhibit the most tolerant of potential fouling organisms. For cuprous oxide formulations, a continuous release rate exceeding 10 µg Cu/cm2/day is necessary for effectiveness. The challenge for antifouling paint chemists was therefore to have both a durable paint matrix that could slowly release biocide, and to have biocides that were:
- toxic to fouling organisms, but not toxic to applicators or non-target species when released
- stable to enable persistence in the paint, but not so stable that they would not break down in the environment
- broad spectrum across biofouling types, but not so broad as to have a harmful effects in the environment
- leachable, but not too fast, or too slow.
Conventional antifouling coatings are free-association paints in which the biocide particles are physically dispersed through the paint binder. When immersed, seawater penetrates the surface of these coatings and contacts the biocide, which dissolves and migrates to the coating surface by simple diffusion. The two types of conventional coating are ‘soluble matrix’ and ‘contact leaching’ coatings. In soluble matrix coatings the binder, commonly natural wood rosin, is soluble in seawater and fresh biocide is continually released as the binder dissolves. The binder in contact leaching coatings is largely insoluble and biocide release depends on a high enough concentration of biocide particles in the paint film to ensure continuous contact through the film. As particles dissolve at the surface, seawater can penetrate through the micro-channels created to contact biocide deeper in the film. Contact leaching coatings were based on chlorinated rubber of vinylic resin polymers.
The search for more effective biocides transitioned through mercury and arsenic in the early 1950s, subsequently banned on occupational health and safety and environmental grounds, to organotin compounds in the 1960s. The organotins – tributyltin (TBT) oxide, TBT fluoride and triphenyltin fluoride – were found to have broad spectrum biocidal properties and were soon commercialised in antifouling paint products. A further significant technological advance came with the development of organotin copolymer paints in the mid-1970s, in which the copolymer provided both the biocide and acrylic binder for the paint. When immersed, a hydrolytic reaction between seawater and the copolymer caused a cleaving of the TBT from the copolymer, which was released as an active biocide. The residual polymer then changed from insoluble to soluble, with release generating a ‘self-polishing’ effect and constant biocide release rates.
The advantages of TBT self-polishing copolymer (SPC) coatings were an effective life of five or more years (proportional to coating thickness), controllable biocide release rates, ability to overcoat without loss of activity, no depleted coating to be removed before repainting, efficient use of biocide, continuous replacement of active surface, and a self-polishing action that resulted in erosion of peaks of roughness that provided increasing hull smoothness and fuel efficiency with time in use. The fouling problem on ships appeared to have been solved.
However, although TBT compounds were thought to be environmentally safe because of relatively rapid microbial degradation of TBT in seawater to the less toxic dibutyl and monobutyl tins, then to non-toxic inorganic tin, adverse impacts began to show, particularly among shellfish, including commercially farmed oysters. The degradation rate in areas of high input and low water exchange, such as marinas and boat harbours, was insufficient to prevent environmental TBT concentrations impacting on non-target species, particularly highly sensitive molluscan species. When TBT concentrations were detected in whales and dolphins, the die was cast, and the ongoing use of TBT antifouling paints was doomed.
Many countries, including Australia, initially introduced restrictions on the use of TBT paints on small craft, but a global ban on use on all international vessels was subsequently implemented by the IMO International Convention on the Control of Harmful Anti-Fouling Systems on Ships, 2001. The resulting challenge was to find antifouling products that would match the performance achieved with the TBT copolymer antifouling coatings.
Several approaches have been developed. First, for small vessels and ships not needing long antifouling service intervals, the performance of conventional coatings with cuprous oxide or cuprous thiocyanate as the primary active was boosted by incorporating secondary, or booster, biocides and refining the binder properties. Second, TBT-free self-polishing coatings were formulated by incorporating cuprous oxide and booster biocides into silyl, zinc or copper acrylate copolymer or other hydrolysable binders. Third, non-biocidal ‘foul release’ coatings, based on silicone elastomers, were developed. They deter or minimise the adhesive strength of biofouling organisms that results in the sloughing of attached growth when the vessel is underway. Although providing effective solutions, the costs of these new products exceeds that of the almost universally applicable TBT SPC coatings, with an almost magnitude of difference in price between conventional and TBT-free SPC coatings, and TBT-free SPC coatings and foul release coatings.
Another consequence of the TBT saga was the development of a national approval and registration system for biocidal antifouling products. This responsibility lies with the Australian Pesticides and Veterinary Medicines Authority (APVMA), whose primary responsibilities are the approval of veterinary medicines and agricultural chemicals containing active substances. For antifouling paints, any active must be approved to assure quality in manufacture and to ensure human and environmental safety. Then, any product containing an approved active must also be individually approved to ensure quality, safety and efficacy. Approval requires the submission of extensive data packages, and the assessment of even a seemingly straightforward product application can take up to 24 months.
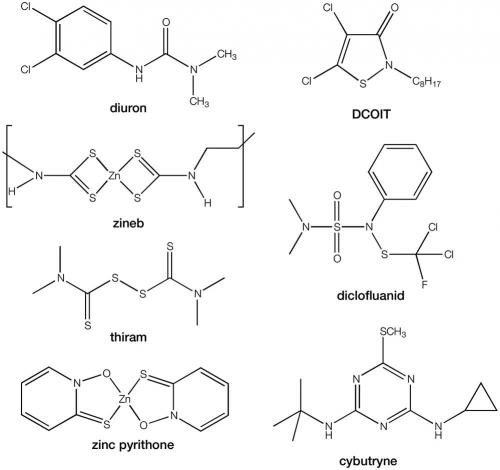
Currently, only 50 biocidal antifouling paints are approved by the APVMA. In these, to supplement copper, only seven booster biocides are approved: diuron, zineb, thiram, dichloro-octyl-isothiazolin (DCOIT), dichlofluanid, zinc pyrithione and copper pyrithione. Copper pyrithione is the only new antifouling active approved during the past 10 years. An application for the approval of cybutryne was submitted in the 1990s, but was rejected when adverse effects began to appear. This biocide has been used in many other parts of the world, but there is now a proposal before the IMO to also ban this under the AFS Convention. In New Zealand, the use of diuron in antifouling paints has been phased out, and thiram will similarly be phased out over coming years.
Effective biofouling management is essential for not only the economic operation of vessels, but also to reduce harmful gas emissions and the spread of invasive marine species by shipping. Some concerns have been raised about the environmental impact of copper released by antifouling paints, but copper remains the mainstay of effective biofouling management. Non-toxic foul release coatings are effective for some types of vessel, but these are not a universal solution owing to cost and operational limitations. The need for antifouling coatings is reflected by the market: the global antifouling paints and coatings market is projected to grow from more than US$5.5 billion in 2015 to more than US$9 billion by 2021. In Australia, where the majority of the market is to small vessels, sales of antifouling paints in the 2016–17 financial year amounted to $17.4 million, up on $16.6 million in 2015–16.
Balancing operational requirements and environmental protection is far from simple when it comes to antifouling.