‘Wine, electrochemistry and song’ was the title of an article I wrote for the December 1996 issue of this magazine. This was all part of the repositioning of my research on wine chemistry after dabbling in environmental chemistry for several years: wine was a much more attractive medium for study. I was ‘converted’ to electrochemistry, perhaps more specifically electroanalytical chemistry, during a period of study leave at the University of Mainz in 1981. As many past members of my research group will testify, it was almost impossible to get through a thesis without some involvement in the joys of electrochemistry. The song part came about as a recently purchased voltammetric system would play ‘I’m forever blowing bubbles’ during the degassing step.
The challenge in determining SO2 in wine from a winemaking perspective is to obtain an accurate measure of the free SO2 concentration. This measure represents the pH balance between molecular SO2 and HSO3–, the former being the critical value for the antimicrobial action. SO2 may be bound to various wine components and this bound concentration plus the free concentration gives the total, a value often regulated in many countries. Just to complicate matters, some SO2 in red wine is weakly bound to pigments. This is often released in the measurement of free SO2, leading to a clear over-estimate of the actual, or ‘true’ in wine industry jargon, concentration.
When seeking approaches to developing an electrochemical method for SO2, the problem that we faced was the prior removal of dissolved oxygen in the wine. Degassing was not an option because the degassing step would remove some or all of the free SO2. While working on this problem, I was fortunate to be invited to join the PhD advisory team for Guo Nan Chen in the Department of Chemistry at La Trobe University. Chen took up the SO2 challenge with great gusto, completing the PhD requirements in April 1991.
After establishing two flow injection/electrochemical methods, the measurement of the true SO2 concentration in red wine still remained a challenge. This is when Chen came up with the idea of using AC voltammetry. The electrochemical reduction of oxygen is a slow electron transfer and, when using the second harmonic AC mode in particular, there was sufficient discrimination between the peaks for SO2 and dissolved oxygen. Similarly, any interference from anthocyanin pigments is minimised in the second harmonic AC voltammetric approach. A comparison of the results between the AC method and a spectrophotometric method (the only check method available at the time) was sufficient to validate the AC voltammetric approach in measuring the true free SO2 in red wine (Analyst, 1991, vol. 116, pp. 253–6). Despite my best endeavours at the time, I was unable to convince the wine industry or instrument manufacturers to develop a commercial instrument based on Chen’s work.
There have been rapid advances in electrochemical equipment in the 20 years since Chen did his thesis work. Disposable screen-printed electrodes are now readily available, which are much easier to use than the dropping mercury electrode when I started in this field. There have also been significant advances in simplifying the size and cost of potentiostats.
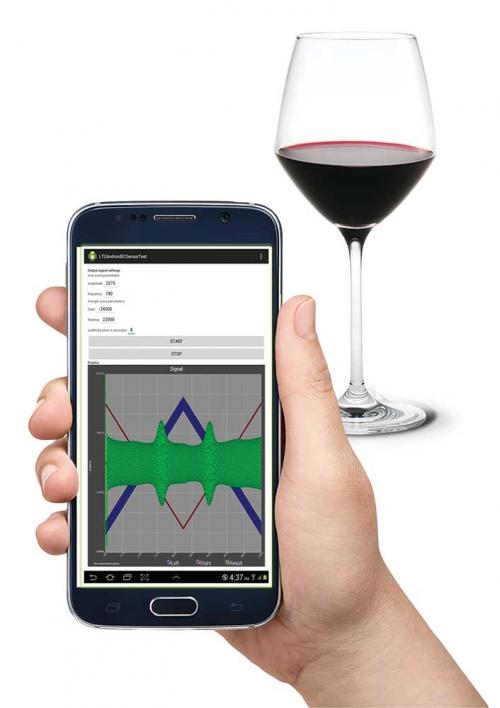
This is where Associate Professor Conor Hogan FRSC, FRACI CChem, and Darrell Elton from La Trobe University enter the story. After demonstrating the capacity of utilising a mobile phone for electrochemiluminescence (ECL) detection, the researchers then turned their attention to adapting a mobile phone for ‘good old-fashioned electrochemistry’ (to use their term). It turns out that within the mobile phone electronics, there is sufficient diversity that it can be used as an analytical instrument. In the ECL procedure, the audio output can generate an applied voltage to the sample and the resulting luminescence can be collected by the phone’s built-in camera.
For an electrochemical measurement, the audio output applies the voltage that Conor tells me consists of ‘an AC perturbation superimposed on a triangular waveform’. The resulting oxidation or reduction current can then by monitored utilising the microphone connection of the phone. The current/potential output is mathematically treated to generate what ones expects for a second harmonic AC voltammogram with peak area related to concentration. This simple and elegant approach is now under patent (WO 2017/156584 A1).
The image gives an indication of what the system looks like – all that is missing are the electrodes. Having seen the system in operation for assessing SO2 in wine, I was amazed at the simplicity of its operation, and while the technology is still at the proof-of-concept stage, there is every possibility of its success in measuring the true free SO2 in red wines in a rapid and easy-to-use way.
The ‘ElecTrobe’ project, as it is known, has received considerable funding from La Trobe University’s Strategic Innovation Fund as well as Wine Australia. Collaboration with the National Wine and Grape Industry Centre at Charles Sturt University will provide the necessary wine expertise to ensure that the ElecTrobe measures what is of vital importance in wine production quality control.