On the night of 10 December 2016 in Stockholm, Sweden, the Nobel Prize in Chemistry was given to Jean-Pierre Sauvage (University of Strasbourg, France), Sir J. Fraser Stoddart (Northwestern University, Evanston, Illinois, USA) and Bernard L. Feringa (University of Groningen, Groningen, the Netherlands) ‘for the design and synthesis of molecular machines’. Sauvage was the first to efficiently make a molecule held together by a mechanical bond and a variety of molecular knots. Stoddart was the first to thread a molecular ring onto an axis and Feringa put it all together to make the first molecular motor.
Molecular topology
I have always been attracted to knots. For example, the monkey’s fist (pictured) is a three-dimensional Borromean ring of exquisite topological beauty but also has utility. Hard to tie, but with a symmetry that is easy to appreciate and utility in sailing at the end of a heaving line, rescue rope and as a weight for throwing mooring lines. So too this year’s Nobel Prize in Chemistry. On one level, it is a very traditional prize for synthetic chemistry, but on another level it is a prize for the artistic expression of chemists for creating objects of beauty and utility on the smallest possible scale.
What differentiates chemistry from every other discipline is that chemistry creates its own objects of desire as Berthelot noted in 1860. This science of creation is based on analogies that relate molecules with macroscopic objects, such as switches, cars and knots, because such analogies promote transdiction (inferring invisible events from familiar macroscopic observables has been habitual with chemists since atomism arose in the 19th century) and make it possible to see molecules as objects of manipulation. The essence of this year’s Nobel Prize is the precise manipulation of matter, at a molecular level, into exquisite topological objects. It so happens that these objects also have the potential to change the world we live in through the synthesis of molecular machines and systems and perhaps even the creation of actual artificial life, not just tinkering with existing life as in current ‘synthetic biology’.
The first step towards a molecular machine was taken some time between 1906 and 1912 when Richard Willstätter (Nobel Prize, 1915), while giving a series of seminars in Zürich, postulated the existence of mechanical bonds – the results of interlocked molecular architecture not held together by covalent bands. In 1953, Frisch, Martin and Mark postulated that the unusual mechanical properties of high molecular weight polysiloxanes were due to the existence of interlocking rings, but there was no direct evidence for this.
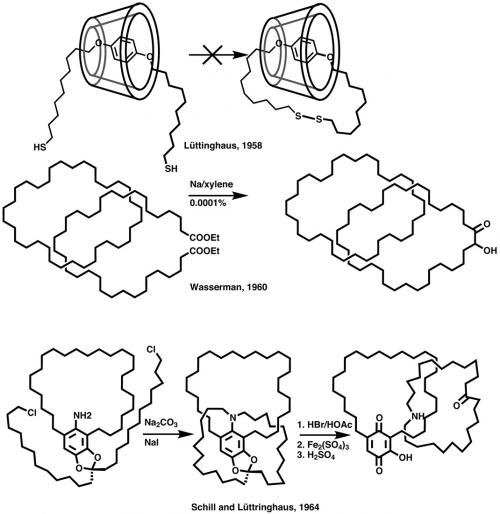
A few years later (1958), Lüttringhaus and Cramer attempted (unsuccessfully) the first synthesis of a catenane (from the Latin catena – chain) by threading a long-chain sulfide through the middle of cyclodextrin and then closing the second ring by oxidising the dithiol to a disulfide. The idea was good, in that the dihydroquinone is about the right size to fit into the cavity of cyclodextrin and should form a stable adduct. It is likely that the expected product was formed but that the analytical methods available in the 1950s were not good enough for isolation of the miniscule amount.
The first [2]catenane was synthesised by Wasserman in 1960 through the acyloin condensation of a dilute solution of a long-chain diester in the presence of a high concentration of a C34 cyclic hydrocarbon. Because this and Lüttringhaus’ synthesis relied on a statistical approach, the yield would be very low (0.0001% in the case of Wasserman) and of no real interest to the chemistry community because the product had no physical or chemical properties of value. However, it is worth noting that this was the first, and remains the only, example of a fully saturated catenane, but more importantly it led to a seminal paper by Frisch and Wasserman (JACS 1961, vol. 83, p. 3789) that discussed ‘molecular topology’ for the first time and ways to directly synthesise mechanically interlocked systems and molecular knots.
The experimental realisation followed shortly (1964) from Schill and Lüttringhaus, who placed an amino group in a C28 macrocyle and did a double intramolecular cyclisation to two alkyl halides to yield a threaded structure, which after oxidative cleavage of the aryl–nitrogen bond led to a [2]catenane in 15 steps. Schill went on to make [3]catenanes, [2]rotaxanes and several molecular knots. Unfortunately, the reaction schemes were often long (20+ steps), low yielding and daunting to carry out. By the early 1980s, fatigue was starting to set in amongst the small number of research groups still interested in mechanically interlocked systems.
It seemed as though there was no solution to the practical synthesis of catenanes when out of the blue a revolutionary solution was published in 1983 in French (Tetrahedron Lett. 1983, vol. 24, p. 5095). Jean-Pierre Sauvage proposed a novel strategy for making catenanes based on the templating effect of copper(I).
This strategy (metal templating) lifted the field from theoretical to real and has been generalised and used by many others to produce new families of topological molecules such as higher catenanes and complex knots.
Jean-Pierre Sauvage
Jean-Pierre Sauvage was born in Paris, France, on 21 October 1944. He studied chemistry at the Ecole Nationale Supérieure de Chimie de Strasbourg and obtained his PhD from the Université Louis Pasteur de Strasbourg (now the University of Strasbourg) under the supervision of Jean-Marie Lehn (1971). Little did he know, but at the time he was already doing Nobel Prize research during his PhD (‘Les cryptates’, Tetrahedron Lett. 1969, vol. 10, p. 2889). This paper led directly to the Nobel Prize for Jean-Marie Lehn, which he shared with Charles J. Pedersen and Donald J. Cram in 1987 for their development of supramolecular chemistry and molecules with structure-specific interactions of high selectivity. Lehn studied chemistry at the University of Strasbourg and did his PhD under Guy Ourisson, did a postdoc for R.B. Woodward (Nobel Prize, 1965) and returned to Strasbourg as a lecturer in 1966. Pedersen had first developed crown ethers to capture alkali metals and Cram extended this to more general host–guest chemistry and Lehn, with his first PhD student (Sauvage), developed the cryptands.
Sauvage managed to publish 10 papers from his PhD, many of them very highly cited. He followed this with a somewhat less successful foray into organometallic chemistry as a postdoc at Oxford with Malcolm L.H. Green (1973–4), from which arose only one minor paper on the synthesis of a half-sandwich ferrocene complex.
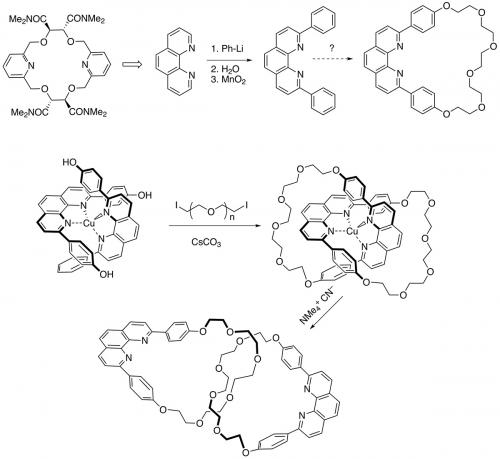
Sauvage returned to Strasbourg in 1974 and continued to work with Lehn until 1982 on the photochemical generation of hydrogen and oxygen (artificial photosynthesis) using noble metal catalysts. In 1980, he also started to work on something he had begun as a PhD student – making polyfunctional crown ethers that could complex transition metals by virtue of incorporation of pyridine rings and tartaric acid. A student, Christiane Dietrich-Buchecker, then discovered the direct arylation of phenanthroline (Tetrahedron Lett. 1982, vol. 23, p. 5291) that opened the door to easy access to 2,9-disubstituted phenanthrolines and now it is not so far to the revolutionary paper of 1983 describing the efficient metal-templated synthesis of catenanes.
The idea was simple: use tetrahedral copper(I) to complex two phenanthroline units that were 2,9-disubstituted with phenols. Addition of a base (caesium carbonate) and a diiodopolyethyleneglycol rapidly led to an interlocked structure. Removal of the copper proved harder than expected due to the ‘catenane effect’ – the first emergent property to be identified in the field of mechanical bonds – but once it was removed very high yields of a functionalised [2]catenane resulted. The structure was proved beyond a doubt in 1985 with an X-ray crystal structure. By removing the copper atom, the phenanthrolines had moved 11 Å – the first example of translational isomerisation.
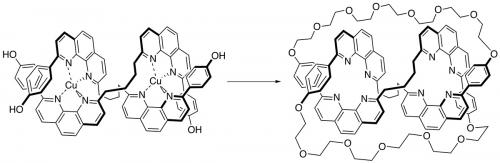
Sauvage went on to use metal templating to make [3]catenates (Angew. Chem. 1987, vol. 26, p. 661), a prototype for the synthesis of high molecular weight polycatenanes, and the first molecule knot – a trefoil, the simplest non-trivial knot being reported in 1989 by Sauvage (Angew. Chem. 1989, vol. 28, p. 189) and a doubly interlocked [2]catenane, the so-called Solomon’s link (J. Am. Chem. Soc. 1994, vol. 116, p. 375). The synthesis of a trefoil effectively crossed the boundary between art and science. Like Eaton’s 1964 synthesis of cubane, Sauvage’s synthesis of a trefoil knot is clearly a result of logic; in the construction of a synthetic strategy, it is also art, not only the simple beauty of the product, but also in the cleverness of the approach. To quote a master of synthesis, E.J. Corey:
The synthetic chemist is more than a logician and strategist; he is an explorer strongly influenced to speculate, to imagine, and even to create. These added elements provide the touch of artistry, which can hardly be included in a cataloguing of the basic principles of Synthesis, but they are very real and extremely important ...
During the 1990s, Sauvage and his research group designed and synthesised a large series of multicomponent transition metal complexes. For example, in collaboration with the Fujita group (University of Tokyo), Sauvage was able to get quantitative yields of [2]catenanes and Solomon’s links using palladium (Fujita) and copper (Sauvage).
The challenge now was to instil utility into these molecules and the first step would require the interlocked systems to move in a controllable way. This was the focus of Fraser Stoddart’s research at Sheffield but also became a focus of Jean-Pierre Sauvage’s group in the late 1990s and 2000s. Sauvage had already demonstrated the movement of [2]catenanes when uncomplexed (see above), and in 1996 he was able to demonstrate an electrochemically controlled translation in [2]catenanes by incorporation of phenanthroline and terpyridine in the macrocycles. Copper(I) tends to complex the two phenanthroline residues in a tetrahedral complex, but when oxidised to copper(II) it tends to an octahedral configuration, switching to the terpyridine ligands, thus creating a controllable motion.
Catenanes, rotaxanes and knots opened the door to new areas of chemistry as well as structurally more complicated motors, machines and nanotechnology. Along with catenanes and rotaxanes, knotted molecules are now commonplace components of integrated molecular machines, often displaying emergent properties that are more than just the sum of their parts.
In common with other great scientists, Sauvage identified a big problem and brought a solution. To do this, he ventured into a chemical wasteland that had been largely forsaken by other chemists since the 1960s and created a niche for himself through the application of transition-metal-templated macrocyclisation to make topological molecules. He was soon joined by hundreds of other research groups that elaborated his original ideas and made beautiful interconnected molecules and even machines.
In the next issue, we will look at the contributions of Fraser Stoddart and Ben Feringa, who took topological molecules, translational isomerisation and photochemical isomerisation and turned them into switches, motors and nanomachines.