The kids are home for the holidays and you want something interesting and educational for them to do – but no stinks or bangs please!
Why not go for surface chemistry? The suggestions here are simple and safe, and the surface science behind them has extremely wide industrial and domestic applications.
Why are plastic bags so pesky to open?
Why do the two sides of new freezer bags stick together and how do you pull them apart so that they can be filled? Enter Johannes Diderik van der Waals.
The van der Waals force is a (generally attractive) force between molecules (and atoms). It is very weak, except at very short distances. It is a force, not a bond like an ionic or a covalent bond. It is this force that holds the individual polymer molecular chains together to form the macroscopic plastic solid such as polythene. To pull the sides apart, you generate a static charge by friction, rubbing the outsides together up and down. Provided the humidity is not too high (which interferes with static electricity), the similar charges repel and, hopefully, the bag opens.
If not, you might try wetting your fingers to stick lightly to each side of the bag to pull them apart.
The first method is generating static electricity;* the second method is a force that holds the plastic to your wet finger.
There are plenty of examples of static effects. The success of the second method –wet fingers pulling the sides of the bag apart – is not often discussed. Tear a small piece of aluminium foil (1 × 2 cm). Place a drop of water on it and then lift and touch it with your finger. It holds tenuously. Metals don’t hold static (much).
The wet patch formed between your fingers and the plastic freezer bag causes strong adhesion. To move away, the surface area of liquid would need to increase and this is resisted. The ultimate explanation has to do with Laplace’s equation.
Repeat the aluminium levitating experiment but use a small drop of oil instead of water. It still works (although probably not as well).
This adhesion holds sand grains together so you can build sand castles; it supresses dust during construction and mining. It can be very strong. Try parting two flat pieces of wet glass by pulling.
How does clingwrap cling?
What does Google tell us how the clinginess works? Many answers attribute it to ‘static’. But the adhesion can’t be static. Same charges repel.
We can wash out the static idea because wet clingwraps work just as well.
Given the slightest opportunity, clingwrap, unlike plastic bags from rolls, clings furiously to itself.
Clingwraps cling better to metal (a conductor of electrons) and glass, but they reject the embrace of plastic utensils.†
So what is in clingwrap and what makes it work? A good start is to up look some patents. Try US4367256A and US5006398. A huge range of clinging agents are incorporated but they are all ‘oily’ and their clinginess depends on liquids in contact coalescing to reduce their surface area (as seen above with your wet fingers opening the freezer bag).
Why does oil spread over water, but water does not spread over oil?
Spreading occurs when the overall surface energy is reduced. Thus, a low-surface-energy liquid will spread over a high-surface-energy solid (or liquid) because it increases the amount of low-energy surface and reduces the amount of high-energy surface. Overall, surface energy is reduced.
Figure out ‘what liquid wets what solid’ – trying both water and oil on various surfaces – and see whether theory matches the practice.
So a rough rule would seem to be that the more readily water wets a surface – i.e. the lower the contact angle and the flatter the puddle (due to the force of gravity, the surface tension and the density of the liquid) – the better the clingwrap should stick.
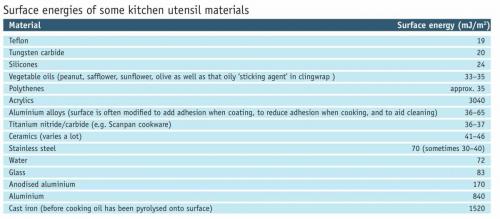
Where there is no overall lessening of the total surface energy, there will be no incentive to cling. This fits with the observation of plastics rejecting the clingwrap’s embrace. Vegetable oils would be expected to wet most surfaces except Teflon, silicones and tungsten carbide.
Why does drawing on a foggy window leave a picture? And why does it come back after the window has dried and fogged up again?
Starting to wear a mask because of COVID-19, I found that my glasses kept fogging up. Then I had a close look at a fogged-up bathroom window on which I had scribbled.
The fogged section has a myriad of tiny droplets spread fairly evenly on the surface; the written section has large globules separated by large sections of clear surface. The act of writing causes an ‘instantaneous’ transition.
Small drops (or small bubbles) have high curvature and thus high surface energy (or high internal gas pressure). Where possible, the high-energy water surface minimises by coalescence. For connected bubbles, the slightly smaller one blows up the slightly larger, until the internal pressures are equalised by equalising the surface curvatures of both bubbles, the smaller one almost flattened (see ‘Bubble politics’, Chemistry in the marketplace, 6th edn, p. 50).
The ghost of your past drawing comes from not cleaning the mirror thoroughly, leaving fatty, soapy material on which the next lot of water vapour can nucleate.
*Two good resources for teachers: www.education.vic.gov.au/school/teachers/teachingresources/discipline/sc... http://people.virginia.edu/~ral5q/classes/phys6263/fall15/Lab01.pdf